Introduction: The treatment of chronic wounds is a serious issue in health care and the development of appropriate wound dressings is a persistent challenge[1]. As clinical studies showed increased pH values in non-healing wounds[2], the shift in wound pH can be an important factor leading to limited wound regeneration. Because traditional wound care does not take the influence of pH into account, applying hydrogels and thus introducing a high variety of chemical modifications might help facing the challenge of long-term wound treatment. As shown in this work the incorporation of functional groups can be used to adapt the material properties to the requirements of wounds. Combined with their good biocompatibility and favorable swelling capacity[3],[4], hydrogels offer promising properties for pH‑regulating wound dressings.
Materials and Methods: We used interpenetrating polymer networks based on poly(ethylene glycol), alginate and pH-active monomers like acrylic acid cross-linked by both ionic interactions and radical polymerization. The materials were characterized for their swelling behavior and buffer capacity in static pH titration. Monitoring cell viability in a colorimetric reaction with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT assay) was used to evaluate the biocompatibility of the hydrogels. Cell response to different pH values and the effect of pH-regulating hydrogels hereon were detected in 2D cell migration assays.
Results and Discussion: By varying the amount of pH-active carboxylic groups, the pH-regulating capacity of the hydrogels could be adapted. The achieved buffer capacity, measured in static pH titration at pH 7.4, covered 1 · 10-5 to 9 · 10-4 mol OH- per gram hydrogel. Furthermore, an accumulation of negative charges in the material led to a high liquid uptake in swelling experiments. The weight increased up to fivefold when incubating the acidic gels in phosphate buffered saline. We also tested the biological suitability for wound dressing applications by evaluating the influence of the gels on cell viability, showing that, according to the MTT assay, none of the hydrogels were toxic. A closer look on pH impact was carried out in 2D wound healing migration experiments. Hereby we tracked two crucial factors of the healing process depending on the pH environment, namely cell migration velocity and the amount of wound closure. Significant migration velocity enhancement and a complete wound closure during the 48 hours of observation could be detected for several gels in comparison to untreated cells under basic conditions (Figure 1).
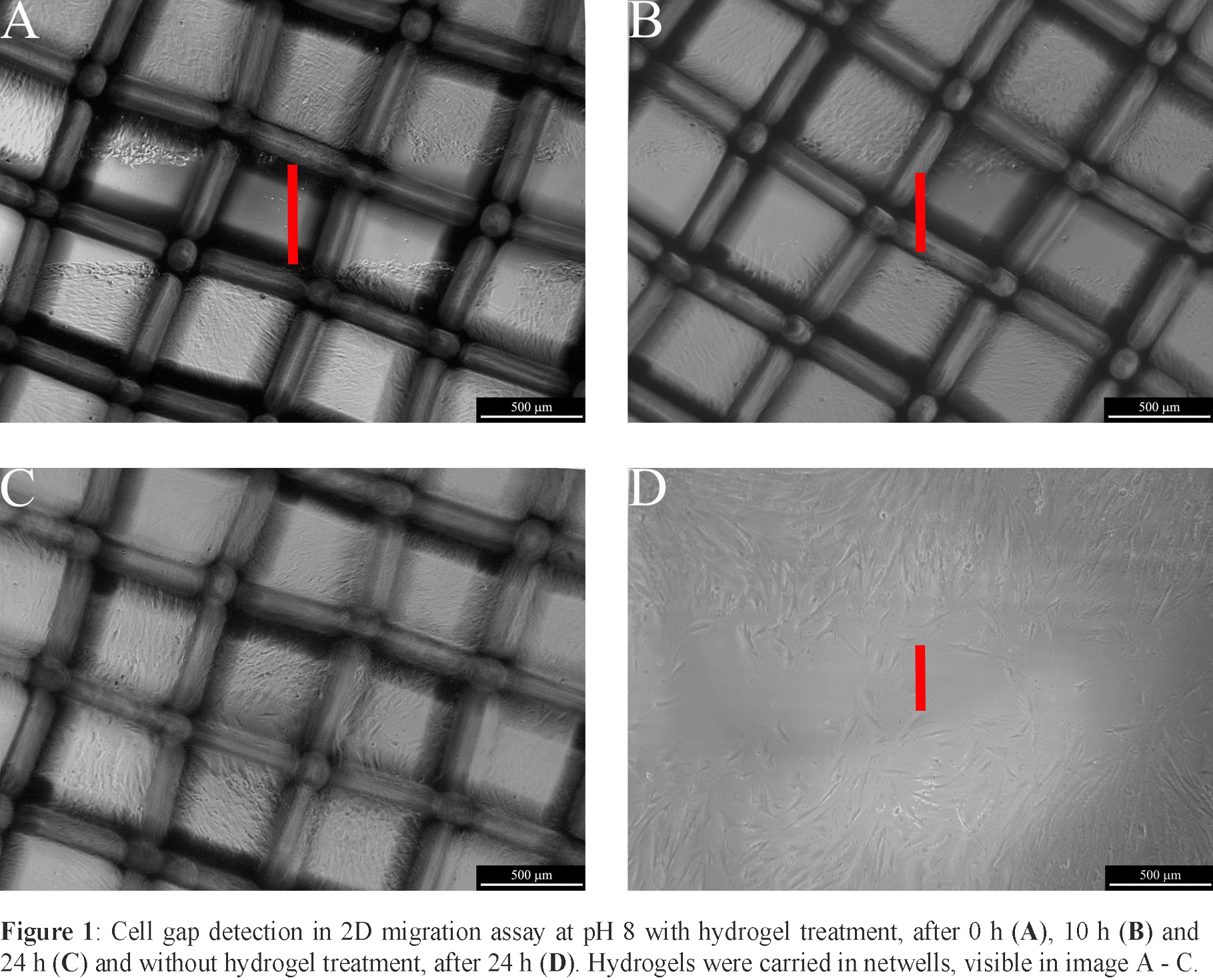
Conclusion: The examined hydrogel systems showed high swelling rates, indicating the ability of good wound exudate uptake, whilst offering a moist healing environment. Due to the incorporation of pH‑active substances, accelerated and complete wound healing could be observed in 2D wound models. Considering all findings we showed that acidic hydrogels are promising biomaterials for wound dressing applications in skin lesions with increased pH values.
References:
[1] C. E. Fife et al., Wounds 2012, 24, 10 – 17.
[2] J. R. Sharpe et al., J. Burn Care Res. 2013, 34, 201 – 208.
[3] S. Rimmer, Biomedical hydrogels – Biochemistry, manufacture and medical applications, Woodhead Publishing Ltd 2011.
[4] J. L. Drury et al., Biomaterials 2003, 24, 4337 – 4351.