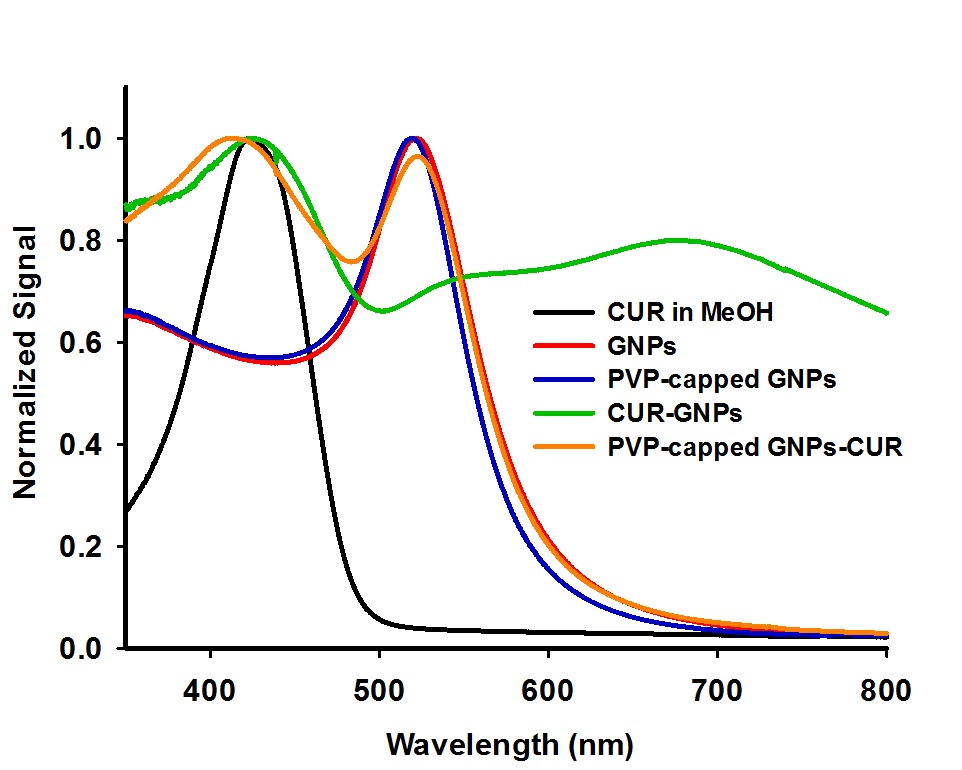
Electrospinning technology has gained considerable interests for a wide range of potential applications, due to simplicity of the processes combined with possibility of large-scale production. Many recent reports on the electrospinning processes to produce extracellular matrix (ECM)-mimetic polymeric nanofibrous matrices provide mounting evidence on the exciting potential for controlled delivery and tissue regeneration applications. An improvement in the controlled delivery of therapeutic drugs, being hydrophobic in nature, with their full potential was attempted by formation of inclusion complexes, i.e., to encapsulate drug as a quest within the matrix of a water-soluble host[1]. In the present study, poly(N-vinylpyrrolidone) (PVP) was electrospun to produce bead-free nanofibrous mat, which is thermally cross-linked and surface-functionalized with 3-(aminopropyl)triethoxysilane (APTES) and finally used as a three-dimensional (3D) substrate material on which curcumin-gold nanoparticles (CUR-GNPs) inclusion complexes would be accommodated. Curcumin is selected as a model drug because it is safe, affordable and efficacious in treating disorders including different types of cancer[2]. Despite its health benefits and safety, the oral bioavailability of curcumin is poor[3], which is mainly associated with its hydrophobic nature and very low aqueous solubility, limiting its potential therapeutic efficacy. Herein, a simple while effective approach to incorporate CUR-GNPs inclusion complexes onto the surface of APTES amine-modified PVP nanofibrous mat (NH2-CL-PVP) was demonstrated.

The two-step method to fabricate NH2-CL-PVP nanofibrous mat incorporating CUR-GNPs inclusion complexes (hereafter referred to as CUR-GNPs@NH2-CL-PVP) included (i) synthesis of aqueous colloidal solutions of GNPs by citrate reduction method[4] and CUR-GNPs inclusion complexes by ultrasonication, and (ii) the immersion of NH2-CL-PVP in aqueous solution of CUR-GNPs inclusion complexes under ultrasonication. For comparison purposes, CUR@CL-PVP and CUR@NH2-CL-PVP were also fabricated by immersing nanofibrous mats (CL-PVP or NH2-CL-PVP) in methanolic solution of curcumin under gentle shaking at room temperature for 12 h. The resulted nanofibrous products were characterized by scanning electron microscopy (SEM), thermogravimetric/differential thermal analysis (TG/DTA), attenuated total reflectance Fourier transform infra-red (ATR-FTIR), and X-ray diffraction (XRD) techniques. On the other hand, UV-Vis, zeta potential and dynamic light scattering (DLS) measurements were conducted for the characterization of aqueous colloidal solutions of GNPs and CUR-GNPs inclusion complexes.
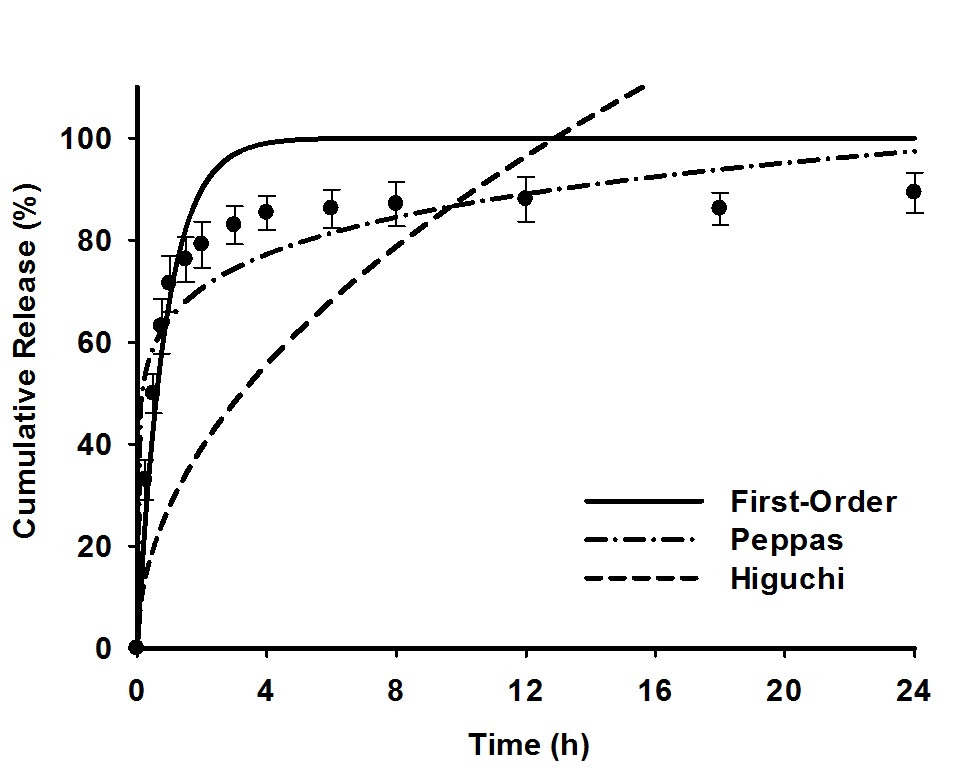
The in vitro release of curcumin from nanofibrous matrices (i.e., CUR@CL-PVP, CUR@NH2-CL-PVP and CUR-GNPs@NH2-CL-PVP) was investigated by two types of release assays, i.e., total immersion and transdermal delivery using a Franz-type diffusion assembly. The release medium was phosphate-buffered saline (PBS, pH 7.4) mixed with a non-ionic surfactant Tween 20 (0.5%, v/v). The biocompatibility of as-prepared nanofibrous scaffolds was evaluated by culturing L-929 mouse fibroblast cells. The adhesion, spreading, and proliferation of viable fibroblasts on different nanofibrous matrices were monitored after 1 to 7 days incubation period (37 oC, 5% CO2) by the MTT colorimetric and lactate dehydrogenase (LDH) assays. Optical microscopy and SEM studies were also taken for morphology evaluation of the cultured L-929 fibroblasts. Finally, the modeling of release data obtained at 37 oC was carried out by non-linear regression fitting to zero-order, first-order, Higuchi and Peppas equations[5] and the results were compared in terms of goodness of the fit. Furthermore, the release kinetics of drug (CUR) from nanofibrous-based systems were also examined in the framework of desorption-limited mechanism from non-degrading substrate.
National Science Council
References:
[1] A. B. Kunnumakkara, P. Anand, B. B. Aggarwal, Cancer Letters 2008, 269, 199-225.
[2] P. R. K. Mohan, G. Sreelakshmi, C. V. Muraleedharan, R. Joseph, Vibrational Spectroscopy 2012, 62, 77-84.
[3] P. Anand, A. B. Kunnumakkara, R. A. Newman, B. B. Aggarwal, Molecular Pharmaceutics 2007, 4, 807-818.
[4] J. Polte, T. T. Ahner, F. Delissen, S. Sokolov, F. Emmerling, A. F. Thunemann, R. Kraehnert, Journal of the American Chemical Society 2010, 132, 1296--1301
[5] P. Costa, J. M. S. Lobo, European Journal of Pharmaceutical Sciences 2001, 13, 123-133.