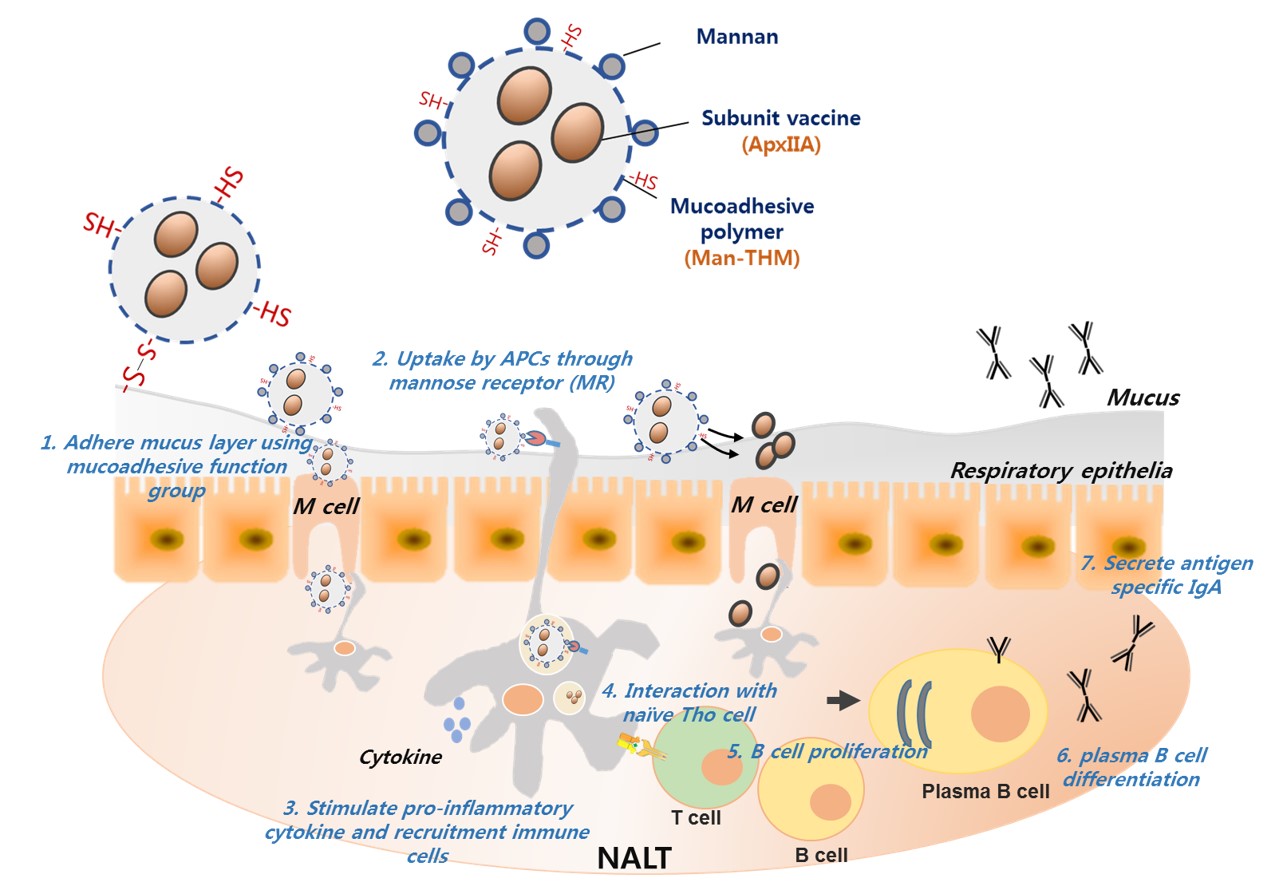
Introduction: The nasal route for vaccine delivery by microparticles has attracted considerable interest, although challenges such as the rapid mucociliary clearance in the respiratory mucosa and the low immunogenicity of subunit vaccine still remain[1]. Recently, a new generation of synthetic polymers known as thiolated polymers, such as thiolated hydroxypropylmethyl cellulose phthalate (HPMCP),have been shown to have strong mucoadhesive properties due to their binding with the cysteine-rich subdomains of mucus glycoprotein in the mucosal site[2]. Here, we applied thiolated HPMCP (TH) to make microspheres as a nasal vaccine carrier for prolonging the residence time in the respiratory mucosa to make interaction time of vaccine molecules and antigen presenting cells (APCs) longer. Moreover, another major hurdle in the development of intranasal vaccines is poor immunogenicity of vaccine subunits due to the lack of “danger signals” that can activate APCs[3]. To this end, we aimed to develop mannan-decorated mucoadhesive THM (Man-THM) as an adjuvant system for nasal vaccine. Mannan is one of the natural PAMPs (pathogen associated molecular patterns) of APCs and is a ligand of the mannose receptor (MR) widely expressed on APCs[4],[5]. Mannan can induce the production of pro-inflammatory cytokines and up-regulate the expression of co-stimulatory molecules in these cells, thereby activating both innate and adaptive immunity[6],[7]. The aim of this work was to explore the potential of a novel Man-THM vehicle for the intranasal administration of ApxIIA as a vaccine against A. pleuropneumoniae which is known to cause contagious porcine pleuropneumoniae.
Materials and Methods: ApxIIa-loaded Man-THM was prepared by the double emulsion solvent evaporation method and using the mixture of 0.25wt.-% PVA and 0.5wt.-% mannan (W2 phase) to decorate the surface of microspheres with mannan.After optimization of the preparation method and characterization of the obtained ApxIIA-loaded Man-THM, the physiochemical properties of the Man-THM as a nasal delivery carrier were evaluated by in vitro and in vivo assays.
Results and Discussion: The surface of the Man-THM was homogeneously coated with mannan due to the mannan’s amphiphilic property, which functions as a stabilizer during the formation of microspheres through W1/O/W2 double emulsion. In a mechanistic study using APCs in vitro, it was found that Man-THM enhanced receptor-mediated endocytosis by stimulating the MR of APCs. In vivo, the nasal vaccination of ApxIIA-loaded Man-THM in mice resulted in higher levels of mucosal sIgA and serum IgG than mice in the ApxIIA and ApxIIA-loaded THM groups due to the specific recognition of the mannan in the Man-THM by the MRs of the APCs. Moreover, ApxIIA-containing Man-THM protected immunized mice when challenged with strains of A. pleuropneumoniae serotype 5.The number of bacteria measured in the lung tissue homogenates prepared from mice in the ApxIIA-loaded Man-THM group was approximately 100-fold lower than those from the ApxIIA and ApxIIA-loaded THM groups.
Conclusion: We concluded that ApxIIA-loaded Man-THM enhanced the systemic and mucosal immune responses against ApxIIA, which enhanced the bacterial clearance ability of the mice challenged with A. pleuropneumoniae, thus improving survival. Our results strongly support the efficacy of Man-THM as an adjuvant carrier system for the effective induction of systemic and mucosal immune responses.
This research was supported by the Animal Disease Management Technology Development, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea. (Project code 313014-03); We also acknowledge the National Instrumental Centre for Environmental Management (NICEM) and the National Center for Inter-University Research Facilities (NCIRF).; H.S. Li and S. Marharjan were supported by BK21 plus program.
References:
[1] N. Lycke, Recent progress in mucosal vaccine development: potential and limitations, Nat. Rev. Immunol., 12 (2012) 592-605.
[2] B. Singh, S. Maharjan, T. Jiang, S.-K. Kang, Y.-J. Choi, C.-S. Cho, Attuning hydroxypropyl methylcellulose phthalate to oral delivery vehicle for effective and selective delivery of protein vaccine in ileum, Biomaterials, 59 (2015) 144-159.
[3] D. Carapau, R. Mitchell, A. Nacer, A. Shaw, C. Othoro, U. Frevert, E. Nardin, Protective humoral immunity elicited by a needle-free malaria vaccine comprised of a chimeric Plasmodium falciparum circumsporozoite protein and a Toll-like receptor 5 agonist, flagellin, Infect.Immun., 81 (2013) 4350-4362.
[4] F.L. van de Veerdonk, R.J. Marijnissen, B.J. Kullberg, H.J. Koenen, S.-C. Cheng, I. Joosten, W.B. van den Berg, D.L. Williams, J.W. van der Meer, L.A. Joosten, The macrophage mannose receptor induces IL-17 in response to Candida albicans, Cell Host Micobe , 5 (2009) 329-340.
[5] H.-L. Jiang, M.L. Kang, J.-S. Quan, S.G. Kang, T. Akaike, H.S. Yoo, C.-S. Cho, The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization, Biomaterials, 29 (2008) 1931-1939.
[6] J. Stambas, G. Pietersz, I. McKenzie, V. Nagabhushanam, C. Cheers, Oxidised mannan-listeriolysin O conjugates induce Th1/Th2 cytokine responses after intranasal immunisation, Vaccine, 20 (2002) 1877-1886.
[7] J. Stambas, G. Pietersz, I. McKenzie, C. Cheers, Oxidised mannan as a novel adjuvant inducing mucosal IgA production, Vaccine, 20 (2002) 1068-1078.