Introduction: Using biomaterials obtained from natural resources is a promising and sustainable approach for applications in tissue engineering and regenerative medicine. The most successful tissue engineered products available today are often made of extracellular matrix components from animals. Keratin has emerged recently as a viable alternative for such applications. It can be readily obtained from human hair, which is abundant, and has demonstrated encouraging bioactive properties. To date, hair keratin templates have been shown to be promising as internal haemostats, for peripheral nerve and bone regeneration and as 3D cell carriers[1].
Materials and Methods: In our group, we are focusing on developing a variety of hair keratin based templates and understanding their properties and capabilities[2]-[6]. For this purpose, we extract keratin monomers in solution from randomized human hair samples under reducing conditions. The keratins were quantified and assessed by Western blotting. Hydrogels were formed by inducing self-assembly through pH moderation. Microporous hybrid sponges were produced by carbodiimide crosslinking of keratin with alginate. Fibrous mats were fabricated by electrospinning of keratin blended with polyethylene oxide in water. The templates were characterized for 1) chemical properties using Fourier Transform Infrared Spectroscopy; 2) mechanical properties by carrying out rheology, tensile testing and flexural testing; 3) biocompatibility by carrying out fibroblast cultures. Preliminary in vivo trials were conducted via subcutaneous implantation of the various templates into wild-type mice, over 4 weeks.
Results and Discussion: The keratin hydrogels produced were stable and could support fibroblast encapsulation and proliferation in 3D while reducing construct contraction in comparison to collagen type 1 hydrogel (Figure 1A). Mechanical testing and cell culture results showed that in the keratin-alginate sponges, alginate provided structural support while keratin enhanced bioactivity to support cell proliferation (Figure 1B). In addition, the sponges were comparable to commercially available wound dressings in reducing water vapour transmission rate. Submicron sized keratin fibers were produced via electrospinning (Figure 1C). These fibers could be aligned and could support more significant fibronectin deposition by primary human dermal fibroblasts. H&E staining of in vivo sample sections showed that there were no significant acute immunological reactions in all the templates described. The keratin-alginate sponges supported the most significant tissue regeneration and integration compared to commercially available collagen sponges.
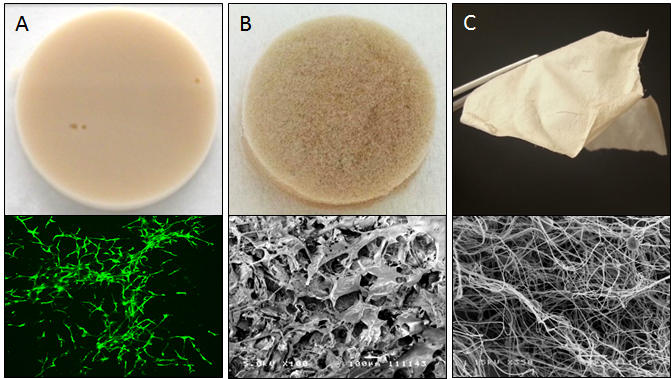
Figure 1. A) Keratin hydrogels were able to support viable proliferation of fibroblasts suspended within (lower panel: Live/Dead staining). B) Freeze-dried keratin-alginate sponges exhibited highly porous microarchitecture (lower panel: SEM). C) Electrospinning keratin-PEO blends produced fibrous mats made up of submicron diameter fibers of consistent morphology (lower panel: SEM).
Conclusion: In summary, human hair keratin is a viable source of biomaterial derived from an abundant natural resource. Different forms of keratin based templates can be produced easily and these have the potential to be developed into functional templates for tissue engineering and regenerative medicine applications.
Ministry of Education Academic Research Fund Tier 1 (RG42/13)
References:
[1] Rouse and van Dyke. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 3:999-1014. 2010.
[2] Wang et al. Human Keratin Hydrogels Support Fibroblast Attachment and Proliferation In Vitro. Cell and Tissue Research 347(3) : 795-802. 2012.
[3] Taraballi et al. Understanding the Nano-topography Changes and Cellular Influences Resulting from the Surface Adsorption of Human Hair Keratins. Adv Healthcare Mat 1(4):513-519. 2012.
[4] Sow et al. Electrospun human keratin matrices as templates for tissue regeneration. Nanomedicine (Lond) 4:531-41. 2013.
[5] Wang et al. Culturing Fibroblasts in 3D Human Hair Keratin Hydrogels. ACS Applied Materials & Interfaces 7(9):5187-98. 2015.
[6] Hartrianti et al. Modulating Mesenchymal Stem Cell Behavior Using Human Hair Keratin-Coated Surfaces. Stem Cells International, Article ID: 752424. 2015.