Introduction: Recent experiments focused on stem cell-substrate interactions using Polydimethylsiloxane (PDMS) widely attribute the lack of cell mechanosensitivity to factors such as substrate noncompliance or amorphous topology[1],[2]. Such studies often directly compare highly hydrophobic PDMS substrate culture results to more hydrophilic substrates like polyacrylamide, although inherent differences in wettability are broadly neglected. Because surface hydrophobicity can affect protein deposition, folding, and ligand activity, we sought to clarify its role as a potentially confounding factor in stem cell mechanosensitivity.
Materials and Methods: We developed and characterized a hydrophilic PDMS-based platform polyethylene oxide-polydimethylsiloxane (PEO-PDMS) with the ability to tune wettability of the elastomeric substrate with otherwise equivalent topology, ligand loading, and mechanical properties. Human bone marrow stromal cells (hBMSCs) were cultured on hydrophobic and hydrophilic substrates of various stiffness covalently functionalized with different ligands (collagen-I, GFOGER and RGD). Difference in collagen assembly was assessed by atomic force microscopy (AFM). Quantitative cell morphology analysis and molecular investigations (after 24h) were performed to evaluate effects on stem cell adhesion and early signalling events. To evaluate the difference in cell traction on the different substrates, we implemented a traction force microscopy (TFM) with embedded fluorescent beads to quantify the substrate deformation.
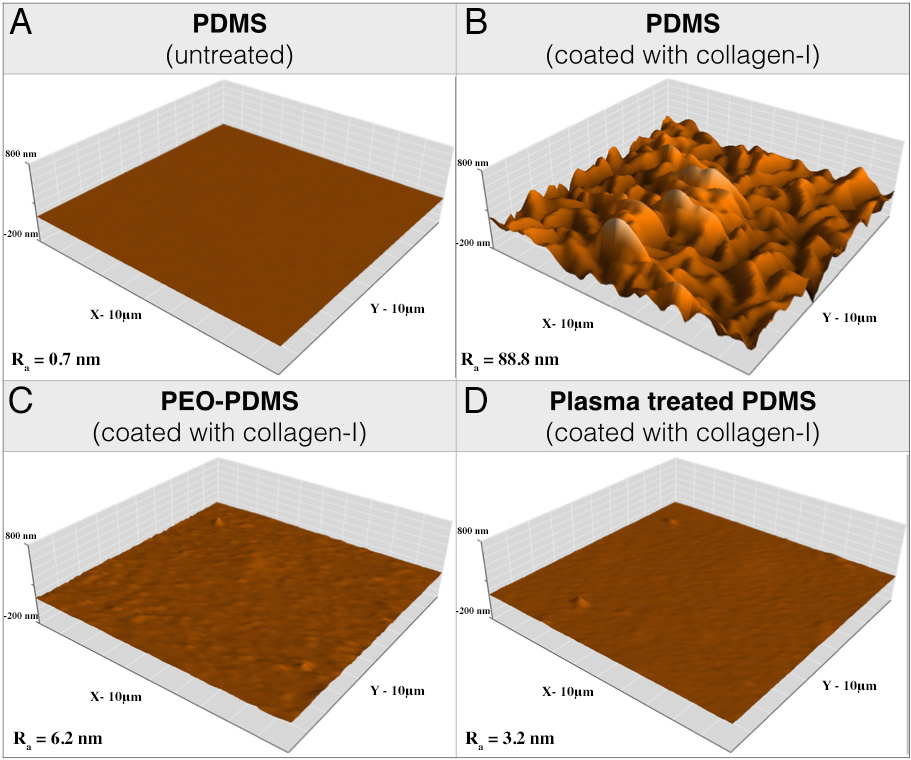
Fig.1: Atomic force microscopy (AFM) images of (A) pristine PDMS and collagen-I monomers coated on (B) PDMS, (C) PEO-PDMS and (D) plasma treated PDMS substrates with same ligand loading (Area size: 10x10um).
Results and Discussion: The first results demonstrate that PDMS hydrophobicity (Fig.1) modulates the nanotopography of covalently bound collagen-I on the surface with a very rough collagen layer on hydrophobic PDMS (Ra = 89 nm) compared to a relatively smooth layer on hydrophilic PEO-PDMS (Ra = 6.2 nm) and plasma treated PDMS (Ra = 3.2 nm). While cells could spread similarly on stiff substrates (2-2.3MPa) for both PDMS and PEO-PDMS coated with collagen-I (Fig.2), cells appeared 2.5-fold less spread on soft hydrophilic substrates (0.65kPa) compared to soft hydrophobic substrates (0.70kPa) suggesting that cells are sensitive to hydrophilic PEO-PDMS stiffness (Fig.2). Molecular investigations reveal lower levels of gene expression of focal adhesion related components on soft PEO-PDMS when compared to soft PDMS consistent with the cell morphology results. The preliminary results from TFM (Fig.3) tend to show that cells also exert less force on the compliant PEO-PDMS (11kPa) – to our knowledge this is the first time that reduced cell spreading has been achieved using soft PDMS substrates.
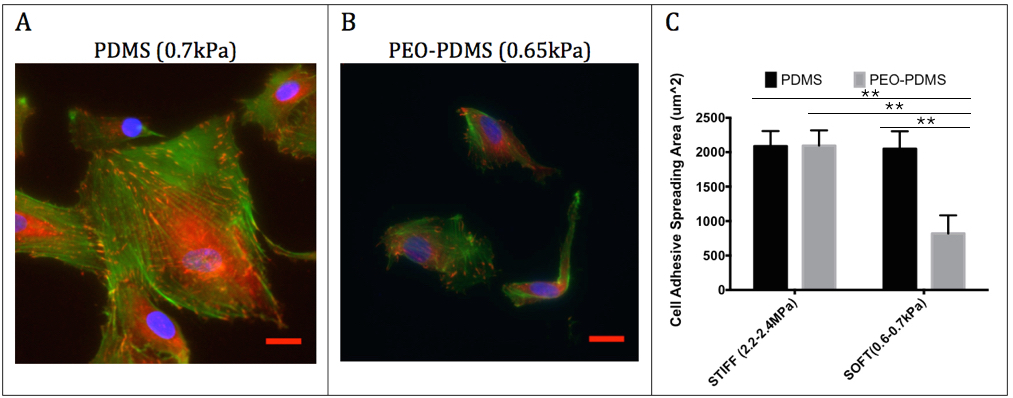
Fig.2: hBMSCs cell morphology on functionalized substrates seeded at 5’000 cells/cm2 after 24 h culture on (A) PDMS (0.7kPa) and (B) PEO-PDMS (0.65kPa) substrates. Cells were stained with FITC-phalloidin (green), DAPI (blue) and immunostained for vinculin (red). Scale bar = 20 μm. (C) Quantitative measurement of cell spreading area after 24 h on PDMS and PEO-PDMS (mean± s.d; n=3; number of cells per sample > 600).
Conclusion: In contrast to previous studies[1],[2], we conclude that stem cells can sense the stiffness on PDMS coated with collagen-I if hydrophobicity is controlled. Therefore, substrate hydrophobicity is a non-neglectable factor that must be incorporated into experimental designs intended to elucidate substrate driven cell response.
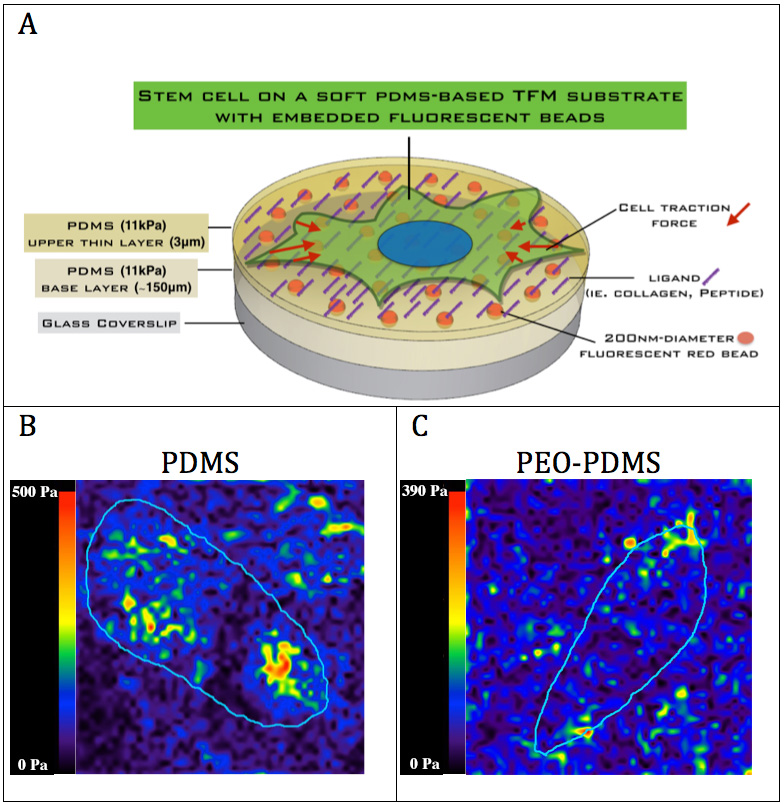
Fig.3: (A) Traction force microscopy (TFM) of hBMSCs on PDMS and PEO-PDMS substrates of similar stiffness (approximately 11 kPa) with embedded 200nm-diameter fluorescent red reporters on (B) PDMS and (C) PEO-PDMS.
Milan Jovic; Aron Horvath; Claude Holenstein
References:
[1] Trappmann, B., et al., Extracellular-matrix tethering regulates stem-cell fate. Nat Mater, 2012. 11(7): p. 642-649.
[2] Wen, J.H., et al., Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater, 2014. 13(10): p. 979-87.