Introduction: While permanent implant materials provide a defined tissue capsule, the responsive tissue volume around a biodegradable metal is less defined due to the diffusion and resorption of degradation products. Moreover, the degradation products could cause negative side effects and initiate a prolonged FBR which may end up in an implant failure[1]. Therefore, biocompatibility has to be proven according to ISO standards, e.g by histological analysis of subcutaneously implanted materials in rats. This well established method has a limitation in the analysis depth of inflammatory cell subtypes and activity. However, detailed knowledge of the time course and the cell subtypes involved in the FBR is essential to describe the immune response and develop appropriate materials. Therefore in this study, a new flow cytometry immune cell panel has been established which allows the quantification of immune cell subtypes by isolating cells from the capsule around biomaterial samples during the first 28 days after implantation.
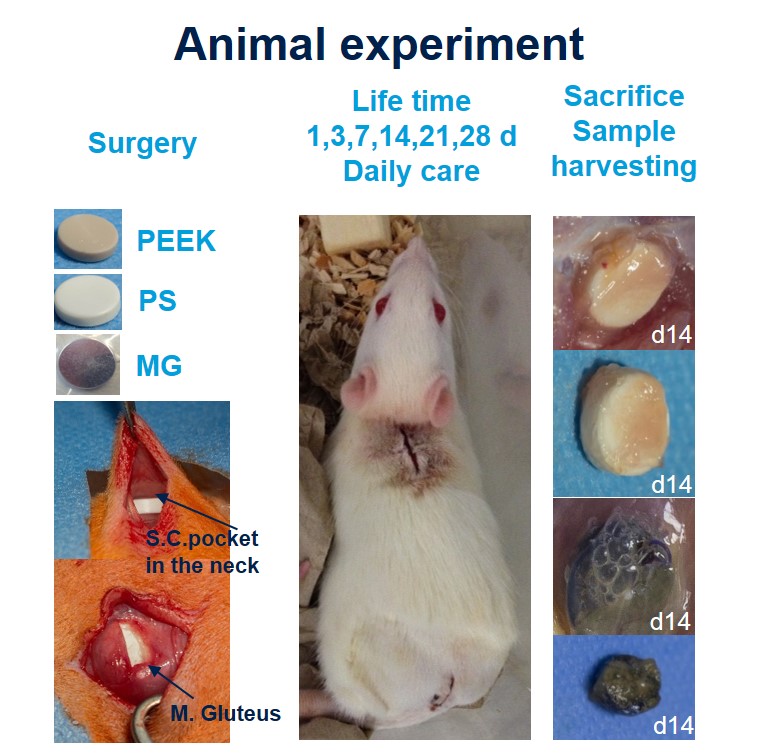
Methods: (Fig 1): Round shaped implants (8mm) made from PEEK (negative control), polystyrene (PS, positive control) or pure magnesium (MG) as a biodegradable material were implanted one intramuscularly (i.m.; M. gluteus) and one subcutaneously (s.c., in the back) of 108 female 12 weeks old Lewis rats. After 1, 3, 7, 14, 21 or 28 days animals were sacrificed (each material n=6) and the capsules which have formed around the implants were harvested. Tissue was minced and digested with collagenase buffer (1h, 37°C) and filtered using different cell strainers. Samples were stained for Dapi (live/dead cells) CD45 (leukocytes), CD3 (T-cells), CD4 (T-helper cells, monozytes, macrophages), CD8 (T cytotoxic cells) and HIS48 (granulocytes, monocytese, macrophages) following the instructions of the manufacturer (eBioscience).
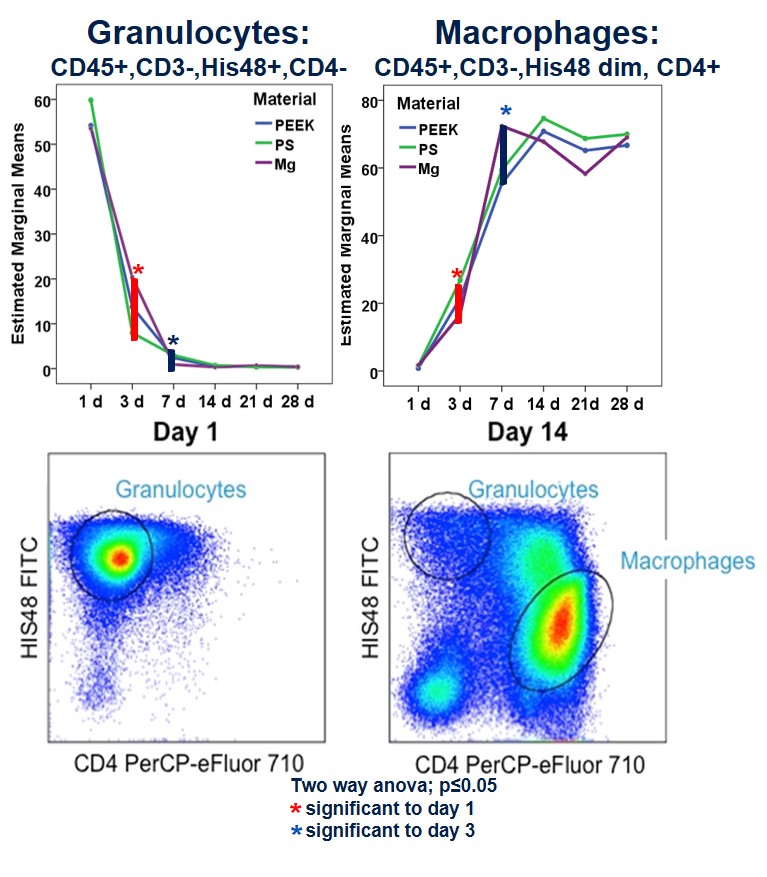
FACS analysis was performed on MACS-Quant. Values of cell subsets are presented as % from CD45+ cells (all leukocytes). Statistics: two-way ANOVA, p≤0.05.
Results: Number of live leukocytes was significantly increased in PS compared to PEEK and MG after day 3 and 7 i.m. and after day 7 s.c. compared to PEEK and MG. Number of live leukocytes was higher in the capsule s.c. compared to the capsule i.m. In general, analysis of the cell subtypes showed significant changes in cell number over time but no significant differences regarding cell composition between the different materials. Granulocytes (CD45+, CD3-, His48+, CD4-) peaked at day 1 in MG, PS, PEEK s.c. and i.m., decreased significantly up to day 3 and further to day 7 and stayed constant afterwards (p≤0.05, Fig. 2). Macrophage number (CD45+, CD3-, His48dim, CD4+) was low after one day but significantly increased up to day 3 and further to day 7 in MG, PS and PEEK s.c. and i.m. (p≤0.05) and stayed constant afterwards. T helper cells (CD3+CD4+) significantly increased up to day 28 in MG and PS s.c. and i.m. as well as in PEEK s.c. (p≤0.003). Number of T helper cells in MG was increased compared to PS and PEEK from day 7. Cytotoxic T cells (CD3+CD8a+) i.m. peaked at day 7 and stayed constant afterwards in MG, PS and PEEK.
Discussion: Our results showed a comparable moderate local foreign body response regarding the cell pattern in all groups with an early immigration of granulocytes to the implant side followed by macrophages und T cells. However, the increased number of live leukozytes found at early time points in the PS group vs. PEEK and MG indicated an increased foreign body response. The degradation of the magnesium did not increase the number of inflammatory cells indicating a good biocompatibility. However, the role of the enhanced T cells in MG compared to PS and PEEK has to be investigated in further studies.
Conclusion: The established flow cytometry panel is a good tool to quantify different immune cell subtypes during the FBR in rats and may provide guidance to future ISO standards in biodegradable metals.
Desiree Kunkel, FACS Core Unit Berlin Brandenburger Center for Regenerative Therapies; German Research Foundation (DFG) WI-2109/10-1
References:
[1] Anderson JM 2001