Introduction: The main challenge in the treatment of cancer therapy is the development of nanocarriers able to selectively transport and release cytotoxic drugs once the target has been reached[1]. Mesoporous silica nanoparticles (MSNs) have emerged as promising candidates to develop targeted stimuli-responsive drug delivery devices for the treatment of solid tumors[2]-[5]. Some specific combinations of cytotoxic agents exhibiting synergistic effects results in the increase of the tumor cell kill capability and the decrease of side effects[6]. Herein we propose the development of a targeted-pH-responsive mesoporous core@shell silica@polymer nanosystem entrapping the antitumor drug doxorubicin (Dox) and incorporating the lectin Concanavalin A (Con-A) in its outermost surface (Fig. 1). The use of Con-A aims at playing a dual role by acting as targeting ligand and therapeutic agent, thus improving the antitumor ability of the nanosystem.
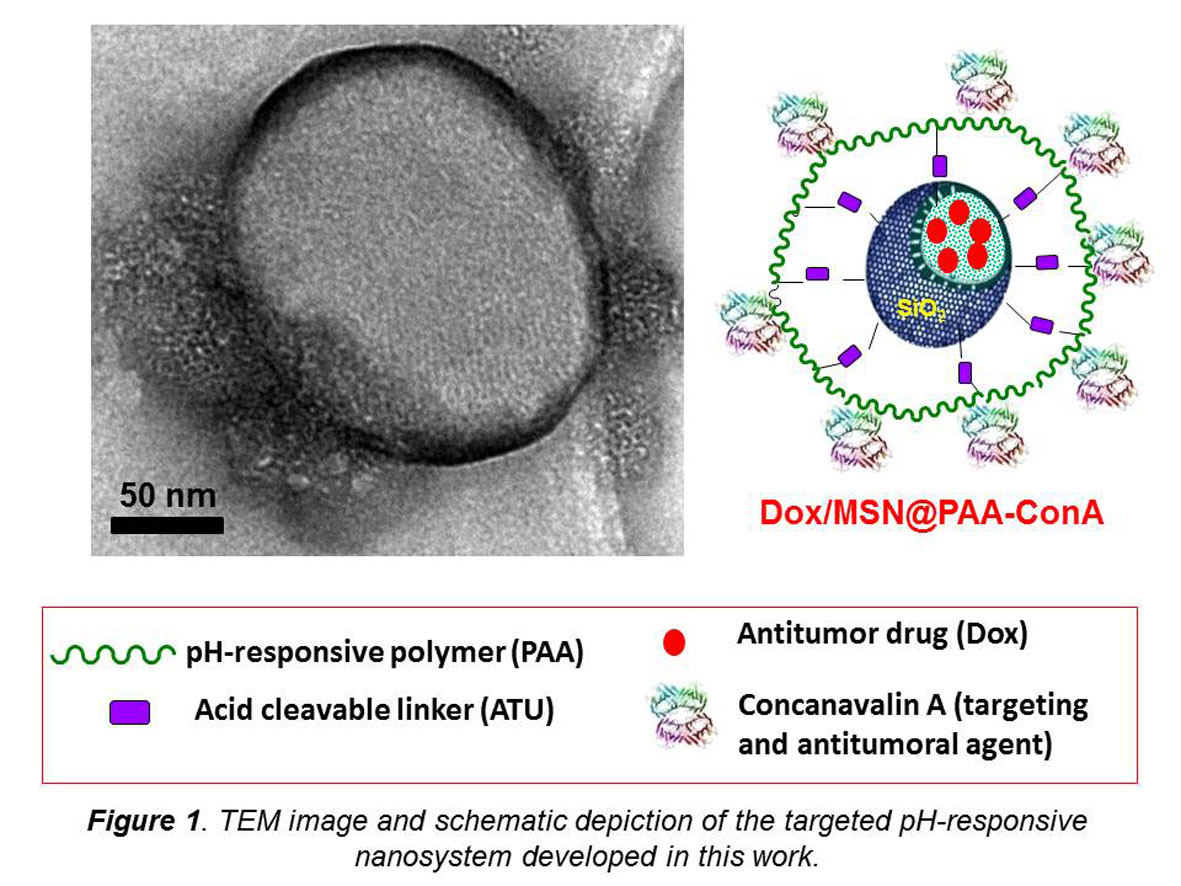
Materials and Methods: Pure silica MSNs were synthetized following the well-known modified Stöber method. Then MSNs were grafter with 3,9-Bis(3-aminopropyl)2,4,8-10-tetraoxaspiro[5,5] undecane (ATU), an acid cleavable linker[7], and loaded with Dox by soaking in a concentrated aqueous drug solution. The capping of the pores was accomplished using a polyacrylic acid polymer (PAA). Con-A was attached to the PAA shell via an EDC/NHS crosslinking method. The materials were characterized by TG, FTIR, 13C solid state NMR, N2 adsorption, SEM, HR-TEM, zeta-potential and DLS. The pH-responsive drug delivery behavior of the nanosystem was tested in vial. Finally, the in vitro behavior of the nanosystems regarding cell internalization and killing capability was evaluated in healthy (MC3T3-E1) and tumor (HOS) cell cultures.
Results and Discussion: The physical-chemical, structural and textural characterization of the materials allowed confirming the successful incorporation of the different molecules to the spherical 2D-hexagonal MCM-41 type MSNs after each synthesis step. The amounts of PAA and Con-A grafted to MSNs were ca. 7% and 10% in weight, respectively, which accounts for the suitability of the functionalization methods here reported. The amount of Dox loaded was ca. 7%. The drug delivery behavior of the full nanosystem evaluated in vial showed that nearly 4-fold higher release was obtained in acidic media than in neutral pH, which demonstrates the pH-responsive drug release ability of the system (Fig. 2A). This finding proves the suitability of the nanosystem for smart Dox delivery system capable of releasing the drug in the acidic environment of the endosomes/lysosomes once it has been internalized by the cell. The in vitro tests indicated that the presence of Con-A as targeting ligand leads to higher nanosystem internalization degree in HOS than in MC3T3-E1 cells. Moreover, the cancer cell killing capability of Dox was synergistically enhanced by the presence of Con-A (Fig. 2B), as derived from cell viability assays.
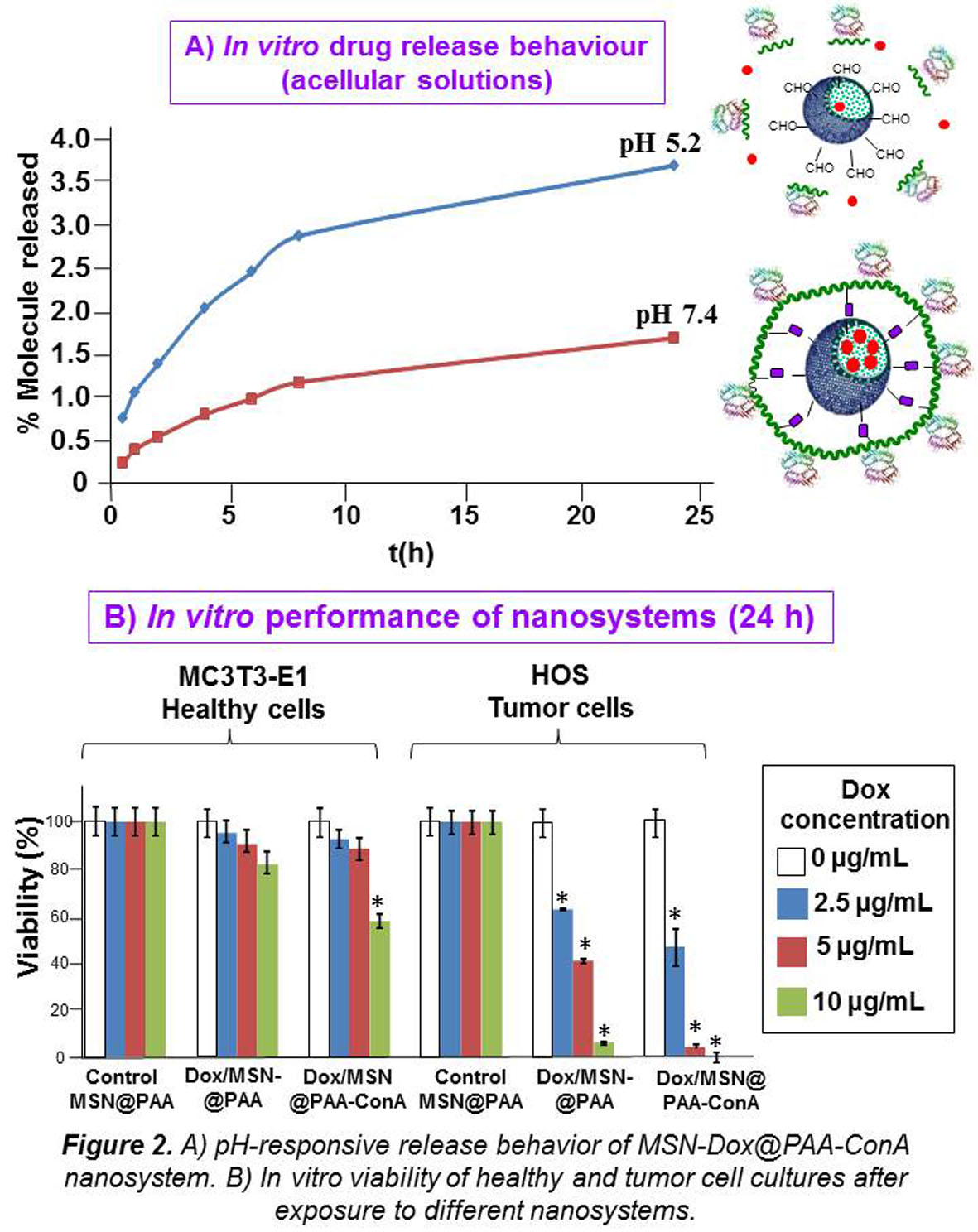
Conclusion: We have developed a novel pH-responsive drug delivery nanosystem incorporating Con-A and Dox. The combination of these two elements into the same nanosystem synergistically enhances its antitumor capacity in a selective fashion. These findings open promising expectations for the further in vivo evaluation of the nanosystems.
Authors acknowledge Ministerio de Economía y Competitividad (MINECO), Secretaría de Estado de Investigación, Desarrollo e Innovación (SEIDI) supporting through projects MAT201235556 and CSO201011384E (Agening Network of Excellence). Marina MartínezCarmona acknowledges Moncloa Campus of International Excellence (UCM/UPM-ISCIII) for a PICATA predoctoral fellowship. Daniel Lozano acknowledges MINECO/SEIDI for a Postdoctoral Juan de la Cierva grant
References:
[1] Langer R. Drug Delivery and targeting. Nature, 1998, 392, 5-10.
[2] Vallet-Regí M, Balas F, Arcos D. Angew Chem Int Ed 2007;46:7548-58.
[3] Colilla M, González B, Vallet-Regí M, Biomater Sci, 2013;1:114-34
[4] Baeza A., Colilla M, Vallet-Regí M, Expert Opin Drug Deliv, 2015, 12, 319-337.
[5] Baeza A, Guisasola E, Torres-Pardo A, González-Calbet JM, Melen GJ, Ramírez M, Vallet-Regí M. Adv Funct Mater, 2014, 24, 4625-4633.
[6] Chow KU, Nowak D, Boehrer S, Ruthardt M, Knau A, Hoelzer D, Mitrou PS, Weidmann E, Biochem Pharmacol, 2003, 66: 711-724.
[7] Chen M, He X, Wang K, He D, Yang S, Qiu P, Chen S, J Mater Chem B, 2014, 2: 428-436.