Introduction: Porous and fibrous scaffolds prepared using various methods are essential components of tissue engineering. Poly(ε-caprolactone) (PCL), due to its biocompatibility and mechanical properties, is one of the most desirable polymers in scaffold preparation for bone tissue engineering applications, and there are many studies on modification of PCL to enhance its biocompatibility. In this study, porous and fibrous scaffolds of PCL containing TCP were prepared by wet spinning and gelatin was immobilized onto it to enhance cell attachment on the surface. In order to increase the local antimicrobial agent delivery to the application site, gelatin microvesicles carrying a model antibiotic were adhered on the scaffold surface. Scaffolds were incubated with human osteosarcoma cells Saos-2 to study its biocompatibility and the antimicrobial effect on E. coli.
Materials and Methods: PCL scaffolds (PWS) and TCP-containing PCL scaffolds (PTWS) were prepared by wet spinning. Gelatin was immobilized on the scaffolds (PTWS-G) by grafting of acrylic acid onto it[1],[2]. Gelatin microvesicles which were prepared by water-in-oil emulsion (w/o) method were loaded with Ceftriaxone sodium and added on the samples via application of vacuum-pressure cycle to prepare the antimicrobial scaffolds (PTWS-G-M)[3]. The release kinetics of the drug from the scaffolds was studied. The effect of Ceftriaxone sodium on E.coli was determined by diffusion tests. Scaffolds were characterized by mechanical tests, scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS).
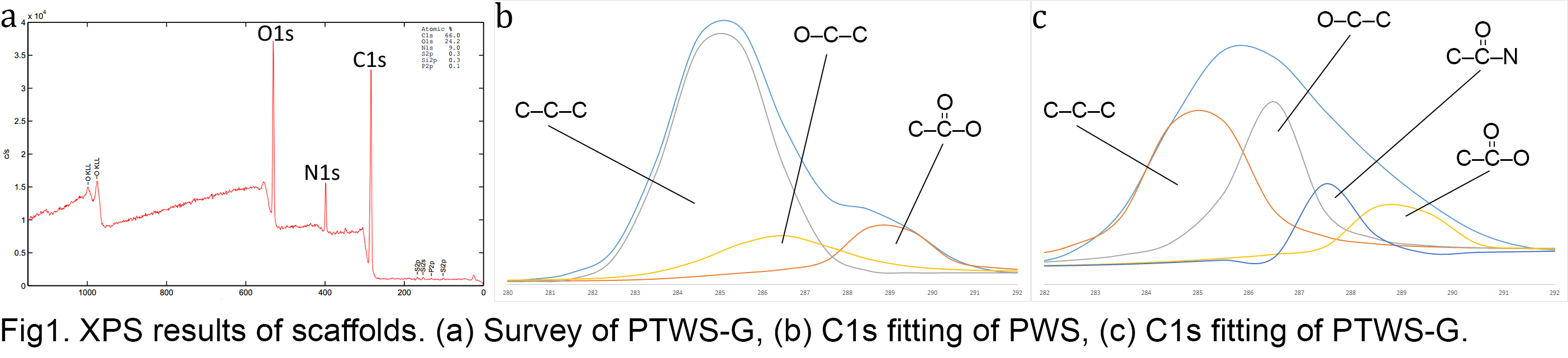
Results and Discussion: SEM images showed that fibers of PWS and PTWS had defect-free, smooth surfaces with average diameters of approximately 200 µm (ImageJ, n=40). Compressive elastic moduli of PWS and PTWS were found to be 190 ± 50 kPa and 350 ± 10 kPa, respectively showing that incorporation of TCP improved the stiffness of PCL scaffolds significantly. EDX showed the presence of TCP on the fibers of PTWS. For gelatin immobilized samples, XPS results showed the presence of nitrogen of the immobilized protein on the surface of PTWS-G, confirming the presence of gelatin. C1s fitting was achieved to show amine bonding (Fig1).
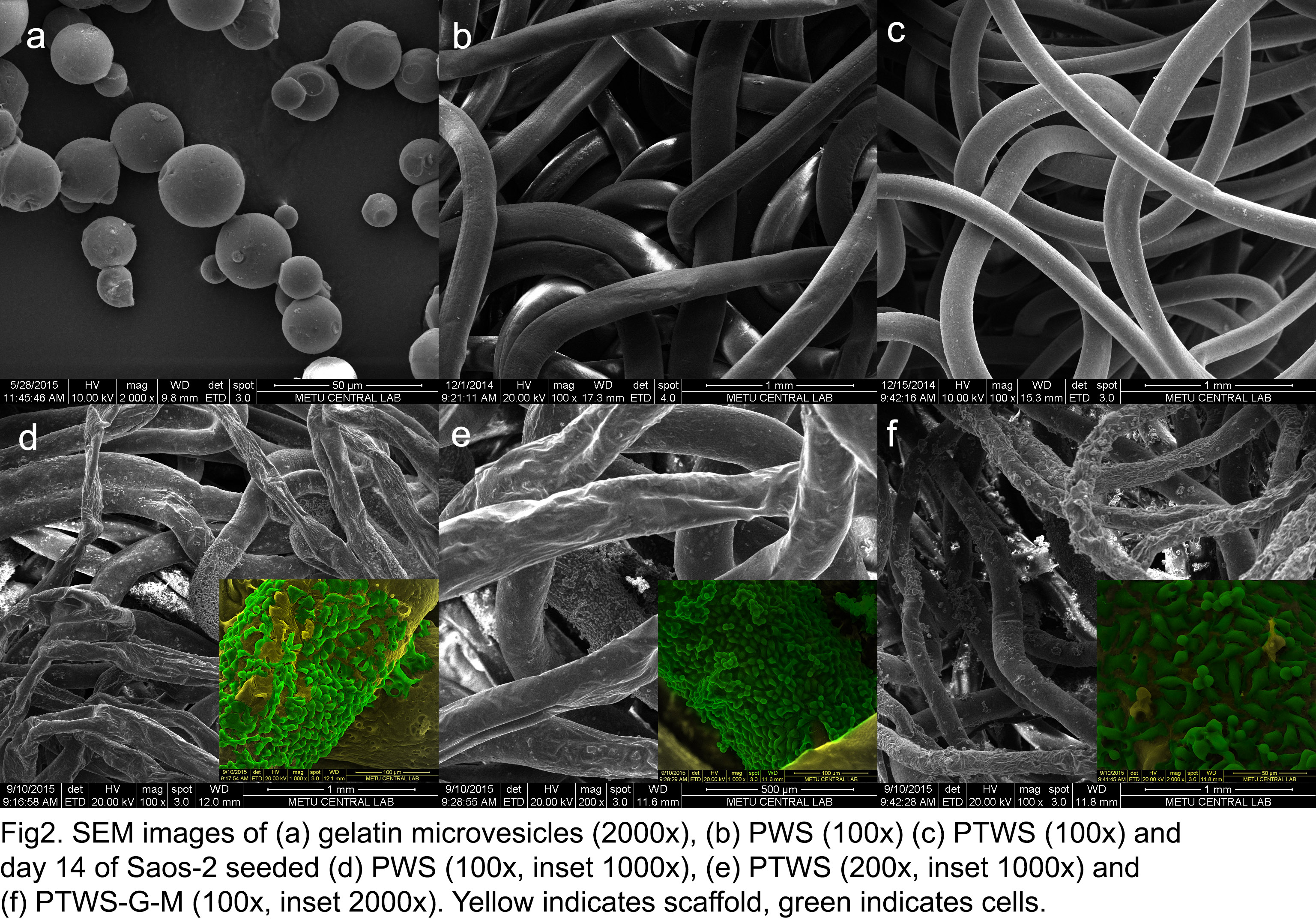
Scaffolds were seeded and incubated with Saos-2 cells. Cells tended to spread on the PCL, and the gelatin immobilization and TCP incorporation enhanced cell attachment and proliferation on the fibers (Fig 2).
Conclusion: PCL scaffolds were prepared by wet spinning and modified with addition TCP, gelatin and antibiotic carrying microvesicles. TCP highly enhanced mechanical properties, gelatin improved cell adhesion and the antibiotic microvesicle system reduced the microbial load. These novel, multi-component and multi-functional scaffolds could be good candidates for bone tissue engineering applications.
Authors are grateful for the Scientific and Technical Research Council of Turkey (TUBITAK) Grant (No 213M708) and METU Grant (BAP-07-02-2014-007-507).; We gratefully acknowledge the support to BIOMATEN through the Ministry of Development funds and by METU.; E.M. is grateful to TUBITAK for his scholarship.; Central Lab of METU for Characterization Analysis
References:
[1] Krishnanand, K., Lal, D., Mishra, A., Gupta, B., Journal of Biomaterials and Tissue Engineering (2013) 3;2(7):233-239
[2] Gupta, B., Krishnanand, K., Deapura, B. L, European Polymer Journal (2012) 48(11):1940-1948
[3] Sezer, U A., Arslantunali, D., Aksoy E A., Hasirci V., Hasirci N, Journal of Applied Polymer Science (2014) 131;8, DOI: 10.1002/app.40110