Introduction: Solid drug nanoparticles[1] (also known as nanosuspensions or nanocrystals) have been shown to be an attractive approach for the delivery of poorly water soluble drugs, with eight clinically approved medicine currently available[2]. Solid drug nanoparticles consist of particles composed entirely of solid drug and therefore have the highest drug loading possible for nanomedicines. This type of nanomedicine has also been shown to give enhanced saturation solubility and increased dissolution rate[2]. Given these characteristics, solid drug nanoparticles offer great potential as drug reservoirs for implanted, sustained release drug delivery devices. However, there are currently two main barriers to administering solid drug nanoparticles alone as a depot injection: firstly the particles will likely migrate from the injection site which may lead to undesired accumulation in non-target tissues; secondly, there will only be very limited control of the rate of drug release from the injection site. Therefore, in order to improve the potential of solid drug nanoparticles for sustained drug delivery, we have developed a novel system in which solid drug nanoparticles are contained within an injectable, polymer matrix. This composite material, which gels upon injection into the body, allows the rate of drug release to be controlled and may prove beneficial in the treatment of long term conditions.
Materials and Methods: Poly(N-isopropylacrylamide) (PNIPAM) nanoparticles were prepared by dispersion polymerisation and characterised by dynamic light scattering and election microscopy. Solid drug nanoparticles of a range of compounds (including HIV drugs such as lopinavir) were prepared by emulsion-templated freeze drying[1],[3] or nanoprecipitation[4]. Nanoparticle/gel composites were formed by mixing the payload nanoparticles and PNIPAM nanoparticles in phosphate-buffered saline and then heating to 37°C. The nanoparticle/gel composites were characterised by rheology and electron microscopy.
Results and Discussion: We have shown that thermally-responsive behaviour of PNIPAM nanoparticles can be designed to form gels under specific conditions. Heating of the PNIPAM nanoparticles above their lower critical solution temperature to 37°C at physiological ionic strength, resulted in the PNIPAM nanoparticles aggregating into a gel. This gel is capable of entrapping up to 40 % w/w a range of payload nanomaterials (including solid drug nanoparticles) to form nanoparticle/gel composites (Figure 1). These nanoparticle/gel composites show controlled drug delivery over sustained periods of time.
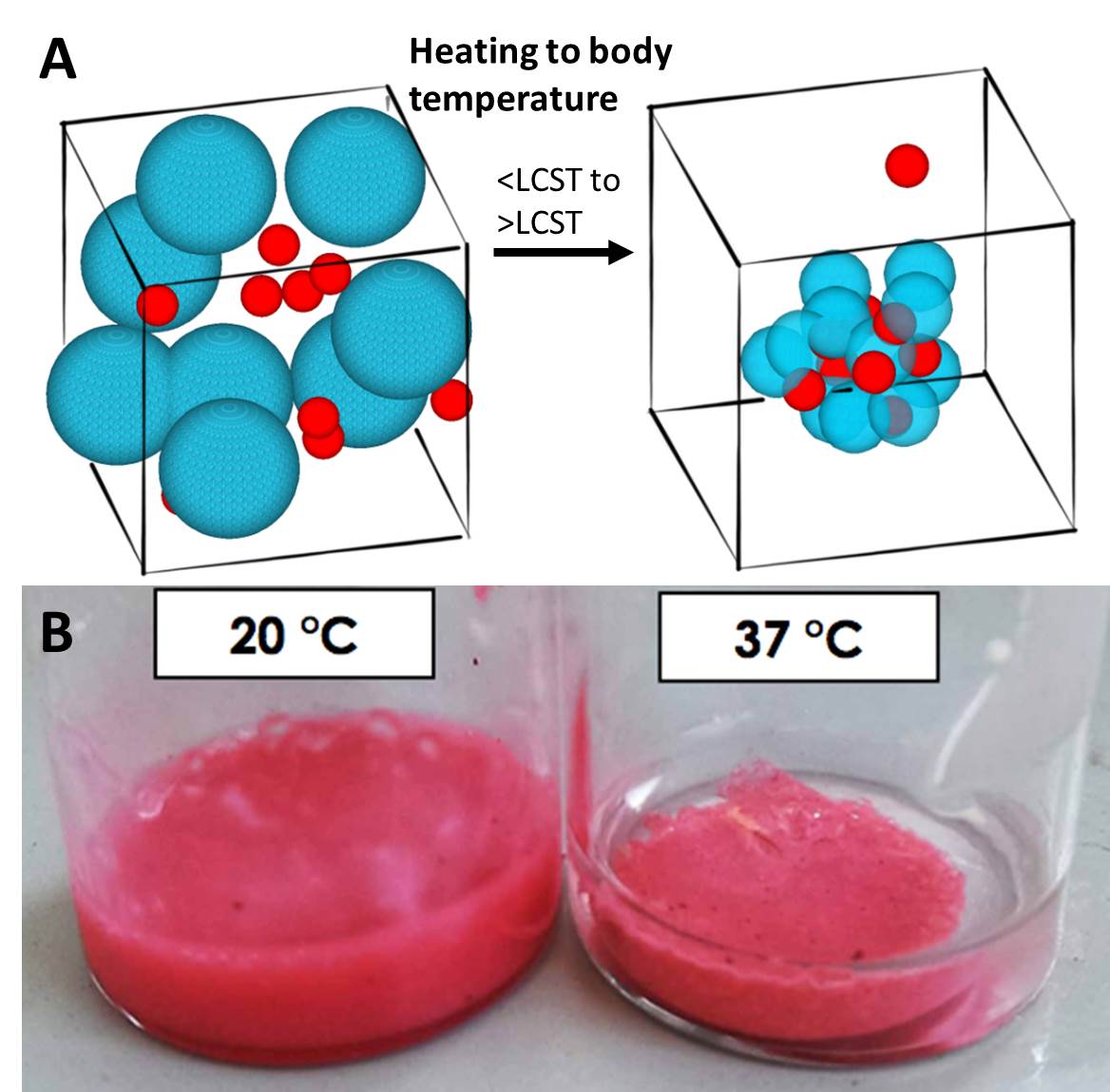
Figure 1. In situ gelation of nanoparticle/gel composites. (a) scheme showing how the heating of the microgel particles to body temperature results in deswelling of the PNIPAM microgels and aggregation of the particles forming a nanoparticle/gel composite (b) photo showing how aggregation of the microgel particles can entrap dye-containing nanoparticles
Conclusions: These nanoparticle/gel composites will offer the potential for sustained release of dissolved drug molecules from an injected depot in the body. Such drug delivery systems may significantly improve patient outcomes for the treatment of long-term conditions, as the greatly reduced frequency of drug administration will avoid issues of poor patient adherence. The ongoing aim of this research is to tailor the system to specific clinical applications.
References:
[1] McDonald, T. O. et al. Adv. Healthc. Mater. 3, 400–411 (2014).
[2] Gao, L. et al. J. Control. Release 160, 418–430 (2012).
[3] McDonald, T. O. et al. Adv. Funct. Mater. 22, 2469–2478 (2012).
[4] McDonald, T. O. et al. J. Mater. Chem. B 1, 4455 (2013).