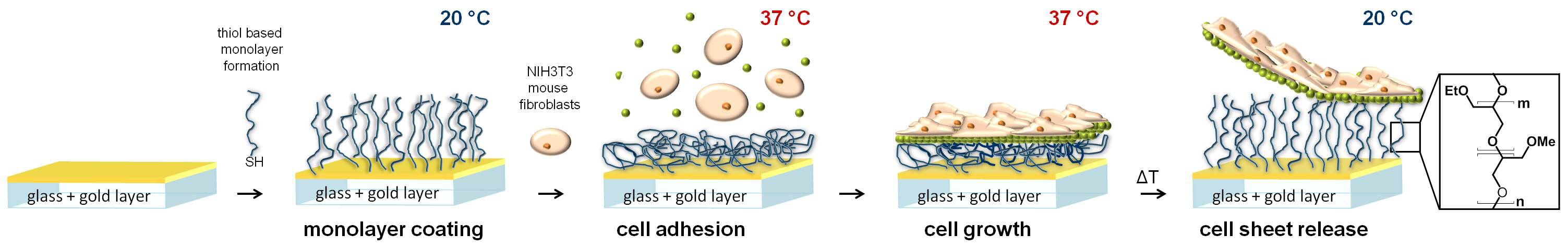
The demand for in vitro grown tissue has attracted significant attention in regenerative medicine within the last decade[1]. Cell sheet engineering is a scaffold-free approach to obtain cell type specific 3D tissue constructs with intact and natural extracellular matrix (ECM)[2]. For this technique a non-enzymatic cell harvest is mandatory to obtain confluent cell sheets with their natural ECM. Surfaces coated with thermoresponsive polymers like poly(N-isopropylacrylamide) (PNIPAM)[3] can be applied to detach confluent cell sheets by decreasing the temperature below the transition temperature of the respective thermoresponsive polymer coating[4],[5]. Such PNIPAM coated tissue culture polystyrene (TCPS) dishes are commercially available for cell sheet engineering purposes. However, the biocompatibility of PNIPAM is still under debate[6]. Poly(glycidyl ethers) composed of methyl glycidyl ether (GME) and ethyl glycidyl ether (EGE) also exhibit thermoresponsive properties and are suitable as support for culturing and detaching adherent cells when tethered to surfaces[5].
Surface coatings of poly(GME)x-stat-(EGE)y were characterized by atom force microscopy (AFM) considering homogenity and Young´s modulus in dry and wet state and swelling at 20°C and 37°C. Chain density of poly(glycidyl ethers) with different thiol anchors on gold surfaces was determined via quarz crystal microbalance (QCM) measurements performing online coatings from ethanol and PBS at 20°C and under cloud point conditions. Switchability of the thermoresponsive surfaces was analysed indirectly by measuring both temperature dependent protein adsorption via surface plasmon resonance (SPR) spectroscopy and adhesion forces between poly(glycidyl ether) functionalized cantilevers and confluent cell layers via AFM. Cell adhesion and cell sheet detachment was monitored via phase contrast microscopy.
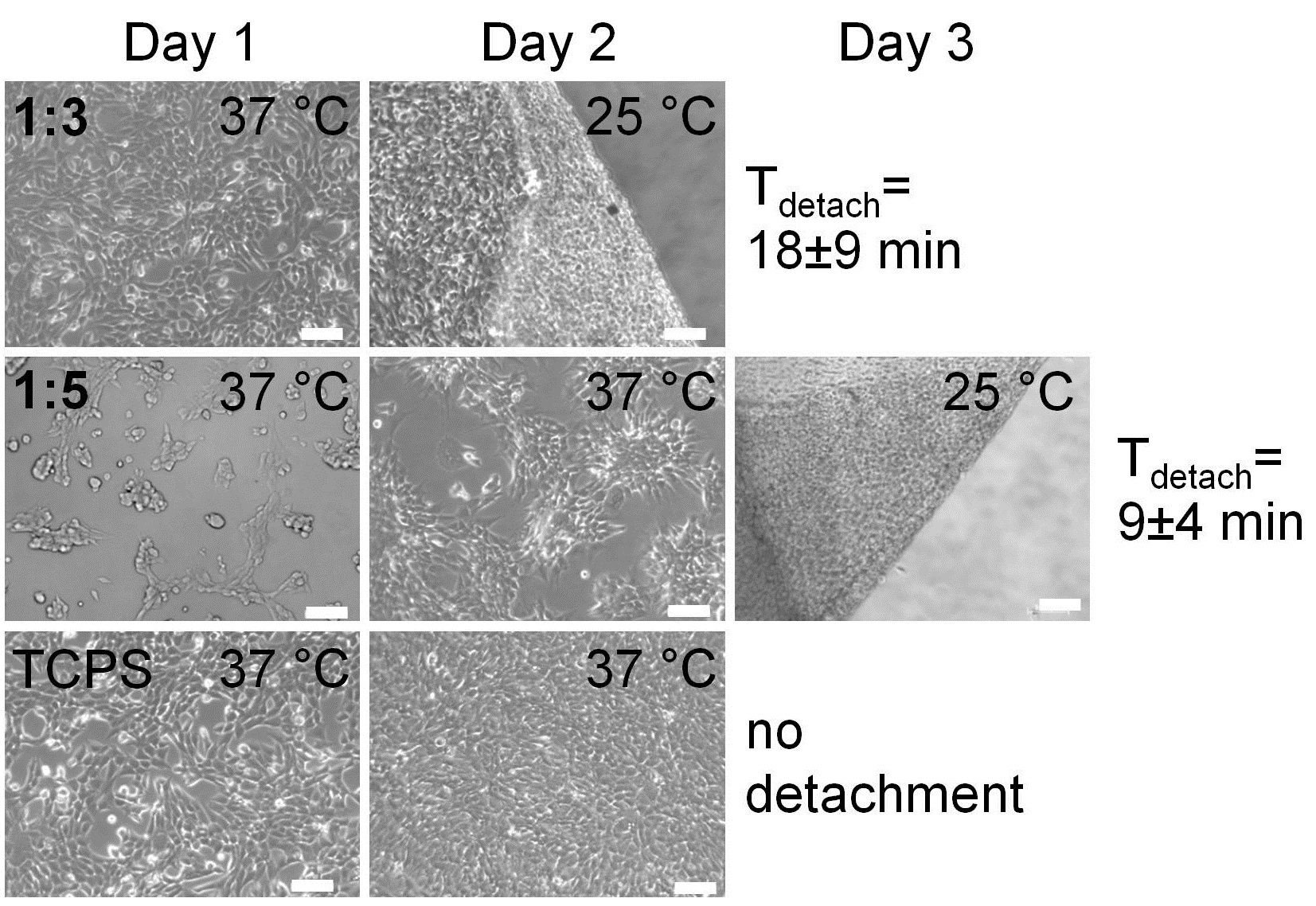
By correlating the chain density on gold surfaces with cell sheet detachment, we observed a threshold value above which successful cell sheet harvest is possible. Both, anchoring groups and coating procedure influence the chain density. Via AFM we observed stronger adhesion forces between poly(glycidyl ether) coatings and confluent cell layers at 37°C compared to 20°C, indicating the switchability of the thermoresponsive surfaces. SPR spectroscopy revealed that the more hydrophobic the respective copolymer (higher EGE content), the more proteins adsorbed onto the surface and the larger the difference in protein adsorption at 25°C and 37°C. Thus, cell adhesion and cell sheet detachment is influenced by the comonomer ratio in poly(GME)x-stat-(EGE)y coatings. The ideal comonomer ratio of GME to EGE for cell adhesion of mouse fibroblasts is 1:3 and allows cells to grow confluent within 2 days. Fastest cell sheet detachment (9 ± 4 min) of NIH3T3 cells was achieved from polymers with a GME to EGE ratio of 1:5[7].
Cell viability was determined by live/dead staining and showed an improved viability for cells harvested from poly(glycidyl ether) based coatings compared to PNIPAM surfaces.
Both, the comonomer ratio within the poly(GME)x-stat-(EGE)y and the chain density are important factors for cell adhesion and cell sheet detachment. Ideal comonomer ratios for NIH3T3 cells are 1:3 for cell adhesion and 1:5 for cell sheet detachment. In addition, successful cell sheet detachment requires a minimal chain density.
S.H. kindly acknowledges the financial support of FCI through a Chemiefonds Scholarship and M.W. is grateful to the BMBF for their support through grand FKZ:13N13523.
References:
[1] R. Langer, J.P. Vacanti, Science, 1993, 260(5110): p. 920-926.
[2] Y. Haraguchi, T. Shimizu, T. Sasagawa, H. Sekine, K. Sakaguchi, T. Kikuchi et al., Nature Protocols, 2012, 7(5): p. 850-858.
[3] H.G. Schild, Progress in Polymer Science, 1992, 17(2): p. 163-249.
[4] J.-F. Lutz, Advanced Materials, 2011, 23(19): p. 2237-2243.
[5] M. Weinhart, T. Becherer, R. Haag, Chemical Communications, 2011, 47(5): p. 1553-1555.
[6] H. Vihola, A. Laukkanen, L. Valtola, H. Tenhu, J. Hirvonen, Biomaterials, 2005, 26(16): p. 3055-3064.
[7] T. Becherer, S. Heinen, Q. Wei, R. Haag, M. Weinhart, Acta Biomaterialia, 2015, in press.