Novel engineering technologies affords unprecedented advances toward medical research. Personalized medicine offers the opportunity to respond to the questions of identifying the right nanomaterial for a particular therapy, reaching the right therapeutic target in the body at the right time, and simultaneously providing feedback as for its efficacy and undesired collateral effects. Nanoengineered biomaterials are of great interest in the context of the drive toward personalized medicine, and may prove to be the necessary catalyst for its large-scale implementation.
In this work, prominent biomaterials, such as nanoporous silicon (pSi) biomaterials are presented and discussed as potential platforms for the individualization of medical intervention; these biomaterials are promising advanced drug delivery technologies for biomedical applications[1]-[8]. It is demonstrated that the surface biofunctionalization of these biomaterials using advanced technologies, such as click-chemistry, microfluidics, etc. Examples on how these materials can be used to enhance the bioavailability of drug/peptide molecules, demonstrating their cytocompatibility, in vivo biocompatibility, intracellular targeting (Figure 1), and theranostic applications, will also be presented and demonstrated. Applications for cancer, diabetes, and cardiovascular diseases of the developed pSi nanocomposites will be discussed and elucidated
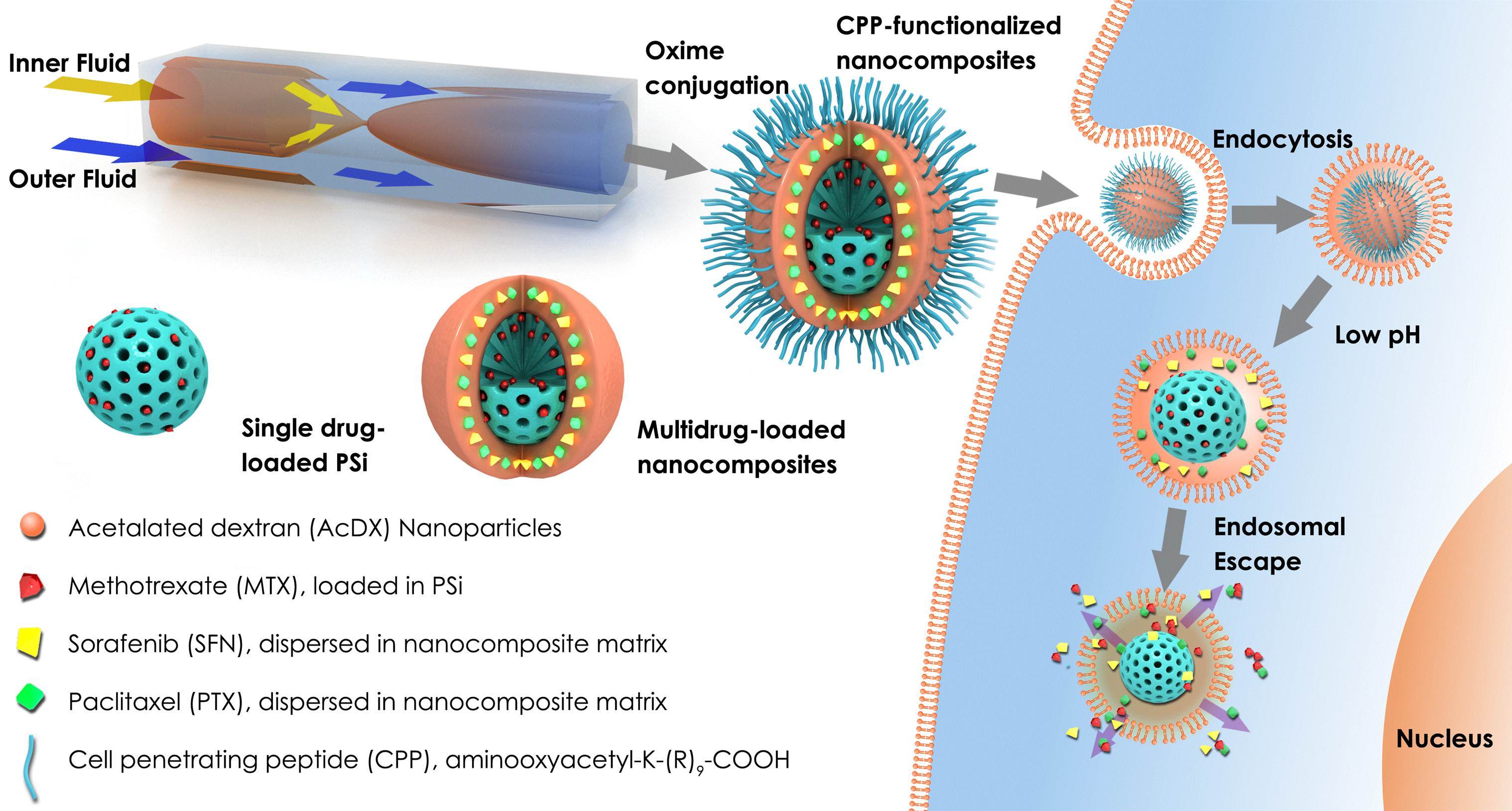
Figure 1: Intracellular targeting of pSi-modified nanoparticles prepared by the microfluidics technology for cancer therapy.
The recent cutting-edge advances on pSi nanomaterials is anticipated to overcome some of the therapeutic window and clinical applicability of many drug/peptide molecules and can also act as innovative theranostic platform and tool for the clinic. The emergence of pSi theranostic systems are expected to convey substantial benefits to the field of drug delivery and cancer/diabetes/cardiovascular therapies as it offers a less invasive alternative compared to the conventional therapeutic strategies and, thereby, enhancing the expectancy and quality of life of the patients.
The Academy of Finland (decisions no. 252215 and 281300), the University of Helsinki Research Funds, the Biocentrum Helsinki, and the European Research Council under the European Union's Seventh Framework Programme (FP/2007–2013, grant no. 310892) are highly acknowledge for financial support.
References:
[1] Araújo F, […], Santos H. A. ACS Nano 2015, 9, 8291
[2] Wang C.-F., […], Santos H. A. ACS Appl. Mater. Interafaces 2015, 7, 2006
[3] Kong F., […], Santos H. A., Hai M., Weitz D. A. Adv. Funct. Mater. 2015, 25, 3330
[4] Shahbazi M.-A. , […], Santos H. A. Nano Res. 2015, 8, 1505
[5] Liu D. […], Santos H. A. Adv. Mater. 2015, 27, 2298
[6] Herranz-Blanco B., […], Santos H. A. Adv. Funct. Mater. 2015, 25, 1448
[7] Wang C.-F., […], Santos H. A. Biomaterials 2015, 48, 108
[8] Liu D. […], Santos H. A. Biomaterials 2015, 39, 249