Polymer hydrogel microspheres as building blocks for extracellular matrices
-
1
McMaster University, Chemistry and Chemical Biology, Canada
Introduction: Synthetic polymer hydrogels can serve as tissue mimetics in somatic andf stem cell research. This talk describes the preparation, properties and initial use of polymer hydrogel microspheres as extracellular matrix components. These microspheres are formed by radical copolymerization of vinyl monomers in near-theta solvents, have tunable surface chemistries and swellabilities in water, and allow formation and confocal tracking of 3D coassemblies with mammalian cells.
Materials and Methods: Typical microspheres are formed by free radical copolymerization of divinylbenzene and 4-methylstyrene with maleic anhydride in a 4:45:50 mol ratio, at a total monomer loading of 2 wt% in a mixture of 70% methylethylketone and 30% acetonitrile, using AIBN as radical initiator, in a method adapted from an earlier procedure to form swellable gel microspheres [1]. The resulting anhydride functional, swellable microspheres are cleaned by centrifugation in tetrahydrofuran, and functionalized by reaction with a range of amines followed by hydrolysis of residual anhydride using 1N NaOH in 1:1 THF/water mixtures. Hydrogel microspheres are suspended in buffered media, co-sedimented with 3T3 and other cells, and studied by fluorescence microscopy.
Results and Discussions: Microsphere formation is by precipitation polymerization similar to those described earlier, but adapted to form highly swellable, transparent hydrogels that are modified with a range of surface chemistries.
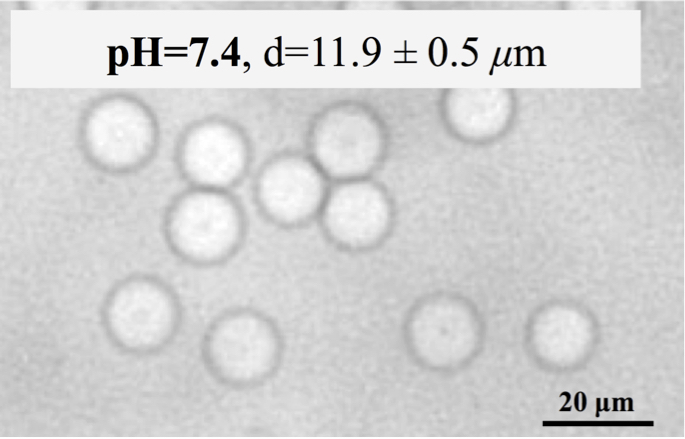
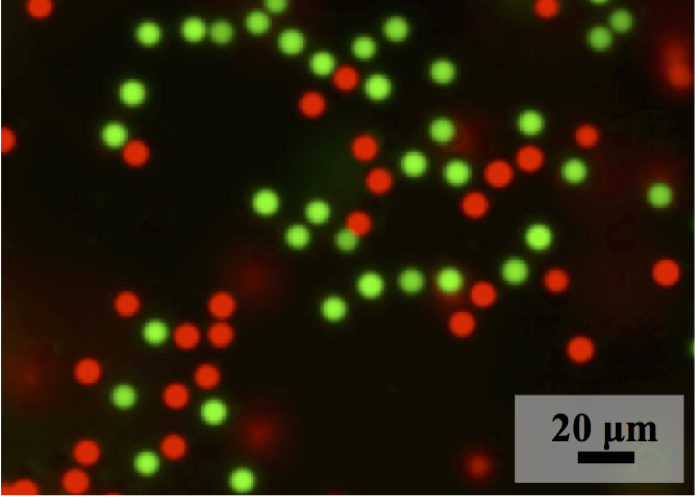
Diameters range from 1 to 25 micrometer, depending on crosslinker content and solvent composition. Hydrolyzed microspheres are highly anionic and swellable, and can be modified by reaction or complexation with a range of mono and polyamines. Reaction with ethylenediamine leads to polyampholyte microspheres, while complexation with polyamines can lead to cationic surface charges. The high transparency permist confocal characterization of 3D assemblies in aqueous media. Radial growth of the microspheres permits inscription of radial composition gradients, and degradable comonomers allow degradation under physiological conditions. Preliminary results of assemblies with 3T3 and other cells will be reported.
Conclusion: Highly swellable, transparent hydrogel microspheres with controllable size, stiffness and surface chemical composition can be prepared in one or two steps from common monomners. These microspheres allow study of polymer-cell interactions using spherical cell mimics in 3D assemblies. Degradable analogs may serve as fugitive structural components in research or injectable ECM environments.
References:
[1] Frank, R. S., Downey, J. S., Yu, K. & Stöver, H. D. H. Poly(divinylbenzene-alt-maleic anhydride) Microgels: Intermediates to Microspheres and Macrogels in Cross-Linking Copolymerization. Macromolecules 35, 2728–2735 (2002).
Keywords:
array,
3D scaffold,
Nano/micro particle,
matrix-cell interaction
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Synthetic scaffolds as extracellular matrices
Citation:
Stöver
H,
Zhao
Y,
Skreta
M and
Zhou
C
(2016). Polymer hydrogel microspheres as building blocks for extracellular matrices.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01459
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Harald Stöver, McMaster University, Chemistry and Chemical Biology, Hamilton, ON, Canada, Email1
Dr. Yuqing Zhao, McMaster University, Chemistry and Chemical Biology, Hamilton, ON, Canada, zhaoy95@mcmaster.ca
Dr. Marta Skreta, McMaster University, Chemistry and Chemical Biology, Hamilton, ON, Canada, skretam@mcmaster.ca
Dr. Christal Zhou, McMaster University, Chemistry and Chemical Biology, Hamilton, ON, Canada, zhouc22@mcmaster.ca