Introduction: The ability to control chemical functionality is an exciting feature of modern polymer science that enables precise design of drug delivery systems[1]. Ring-opening polymerization of functional monomers has emerged as a versatile method to prepare clinically translatable degradable polyesters[2]. A variety of functional groups have been introduced into lactones; however, the direct polymerization of tertiary amine functionalized cyclic esters has remained elusive. In this presentation, we will report a strategy that enabled the rapid synthesis of >130 lipocationic polyesters directly from functional monomers without protecting groups. These polymers are highly effective for short interfering RNA (siRNA) delivery at low doses in vitro and in vivo[3].
Materials and Methods: In the spectrum of delivery systems, polymers have many advantages including tunable structural composition, degradability, and biocompatibility. Yet, they are currently less effective than lipid carriers. To overcome this challenge, we have incorporated key ionizable amines and hydrophobic alkyl chains into polyesters (Figure 1) though lipid design inspiration. We synthesized a library of lipocationic polyesters directly in high yield (>95%), fast polymerization time (~2 minutes), and in gram scale. This was accomplished with precise monomer incorporation ratios to enable tunable hydrophobicity and pKa.
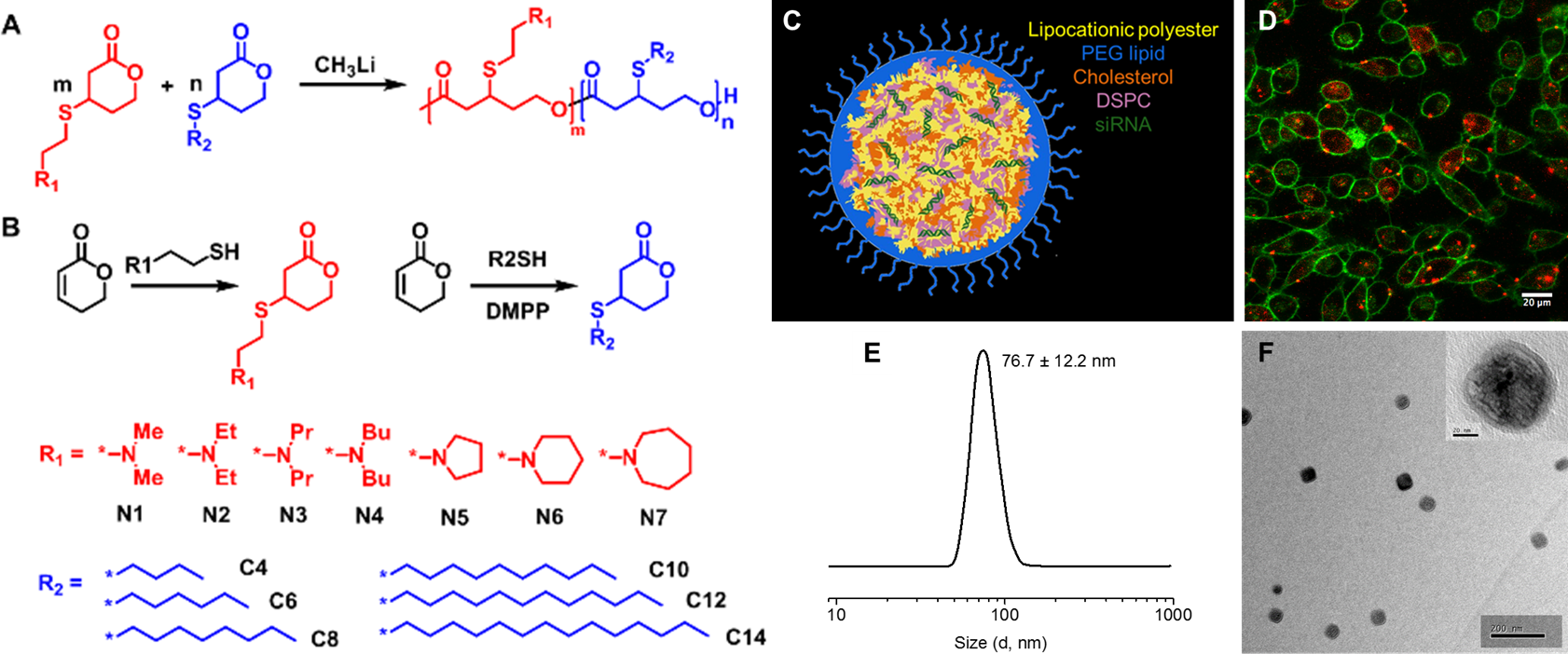
Figure 1. (A) A combinatorial library of 139 lipocationic polyesters was synthesized. (B) Unprotected monomers were synthesized in one step from commercially available 5,6-dihydro-2H-pyran-2-one with aminothiols (N1-N7) and alkylthiols (C4-C14). (C) NP composition scheme. (D) Cellular uptake of Cy5.5-siRNA loaded N1C8 (2:2) NPs. (E) Size distribution measured by DLS and (F) TEM.
Results and Discussion: Delivery efficacy strongly correlated with chemical structure (Figure 2A). Lead polymers enabled >90% silencing at a dose of only 2.4 nM in vitro. RNAiMax was less effective head-to-head (Figure 2B).
Notably, NPs could localize to tumors in vivo after intravenous delivery (Figure 2C). NPs were also able to silence gene expression in tumor-bearing mice, quantified by BL imaging and in tissue lysates normalized against total protein or total tissue amount (Figure 2D).
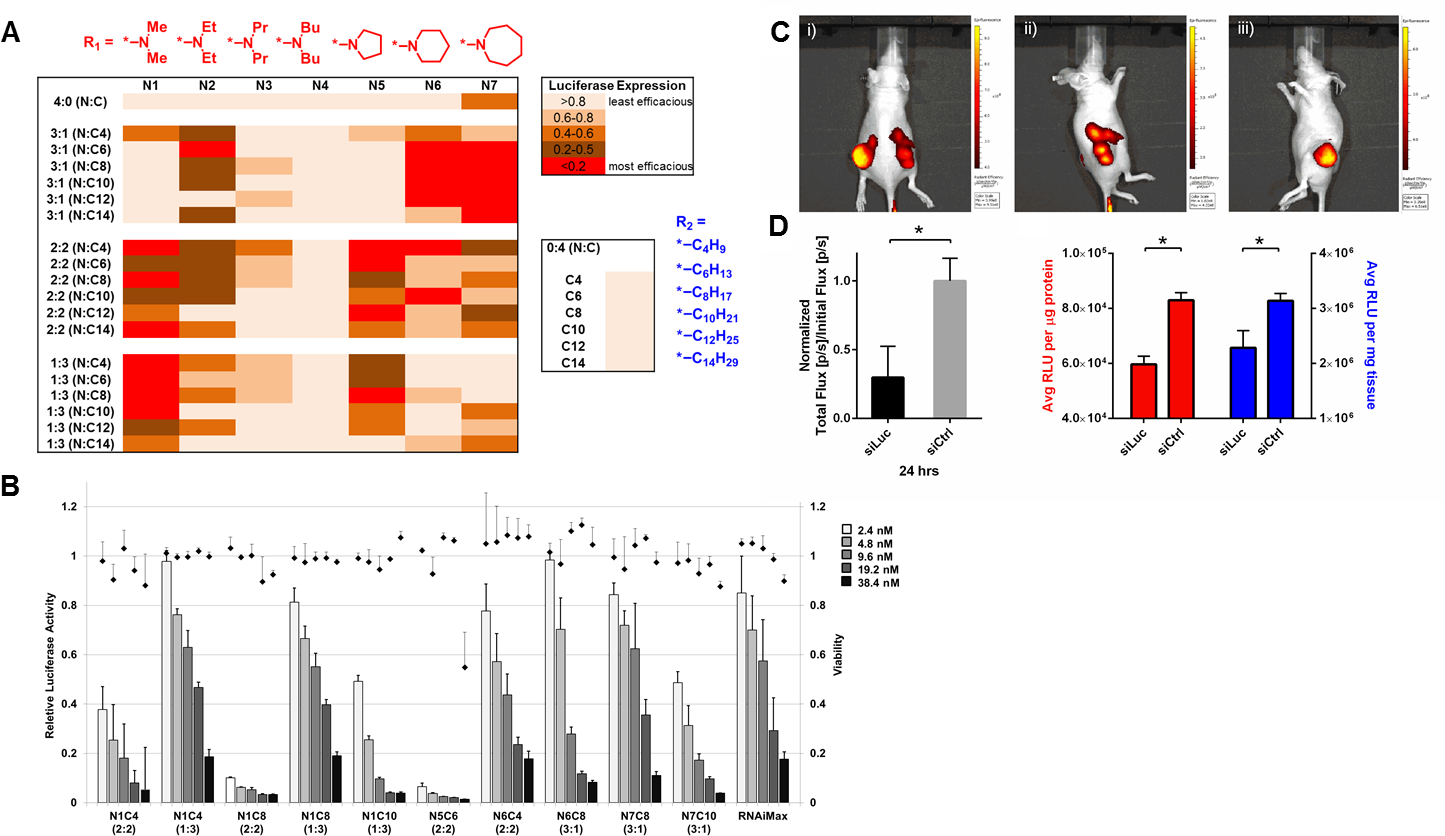
Figure 2. (A) Heat map of in vitro siRNA delivery in Hela-Luc cells. (B) Dose-response of silencing. (C) N1C4 (2:2) NP accumulation in tumor xenografts (IV). (D) Luciferase silencing in tumors (24 hrs, n=4).
Recently obtained data using functional Grignard reagents as initiators will also be presented. This unpublished strategy allowed for fine-tuning of physiochemical properties and correlated to enhanced SAR delivery results. Examination of end group effects provided an additional chemical route to fine-tune pKa, which is an essential component for successful delivery.
Conclusion: Because activity strongly correlated to structure, these synthetic methods provide a versatile way to directly synthesize lipocationic polymers for gene delivery. This new class of lipocationic polyesters is a promising step towards closing the activity gap between lipids and polymers. We envision that the versatility of the chemical methods may allow preparation of functional polyesters for a variety of applications (not only for gene delivery) because nearly any thiol can be used to synthesize functional monomers[3].
References:
[1] Progress in Polymer Science, 37, 237 (2012).
[2] Polymer Chemistry, 1, 260 (2010).
[3] Journal of the American Chemical Society,137, 9206 (2015).