Introduction: Tricalcium silicate (Ca3SiO5, CS) is hydraulic and the hydrate cement (CSC) has been shown bioactive and a potential candidate for bone replacement. CS is thermodynamically stable at 1250ºC -2150ºC. Traditionally, tricalcium silicate powder is synthesized by a solid state reaction, which usually needs a higher temperature and longer calcination time. Furthermore, CS powder made by the solid state reaction often contains a significant quantity of CaO which is harmful to the strength and biological properties of the CSC. The Pechini technique is an alternative, low temperature polymeric precursor route for synthesis of high purity powders.
Materials and Methods: Tricalcium silicate was synthesized by Pechini technique. Firstly, Ca(NO3)2∙4H2O and colloidal SiO2 (40wt% suspension in water) were used as the cation sources. The metal salts with Ca/Si molar ratio 3 were dissolved in the distilled water respectively, and then mixed together with stirring. Citric acid (CA) was dissolved into distilled water with stirring and heating. CA solution was then added into the Ca and Si mixing solution. Then Ethylene glycol (EG) was added into the solution, with a molar ratio EG: CA=2. The mixing solution was stirred continuously at 80-100 ºC in a fume cupboard until the excess liquid was evaporated. The resulting foam was dried at 100-150ºC overnight. The dried gel was then ground to powder and calcined at 1200ºC -1400ºC for 3 h in a furnace. The dried CS powder was crushed to fine powder. The CS powder was mixed with distilled water at different Liquid to Powder ratio (L/P) to make cement samples.
The setting time was measured by Gillmore needle. The SEM, XRD, FTIR were used to characterize the powder and the cement. The concentrations of Ca, P, Si of SBF were tested by ICP-AES. The compressive strength, bioactivity and biocompatibility were also studied.
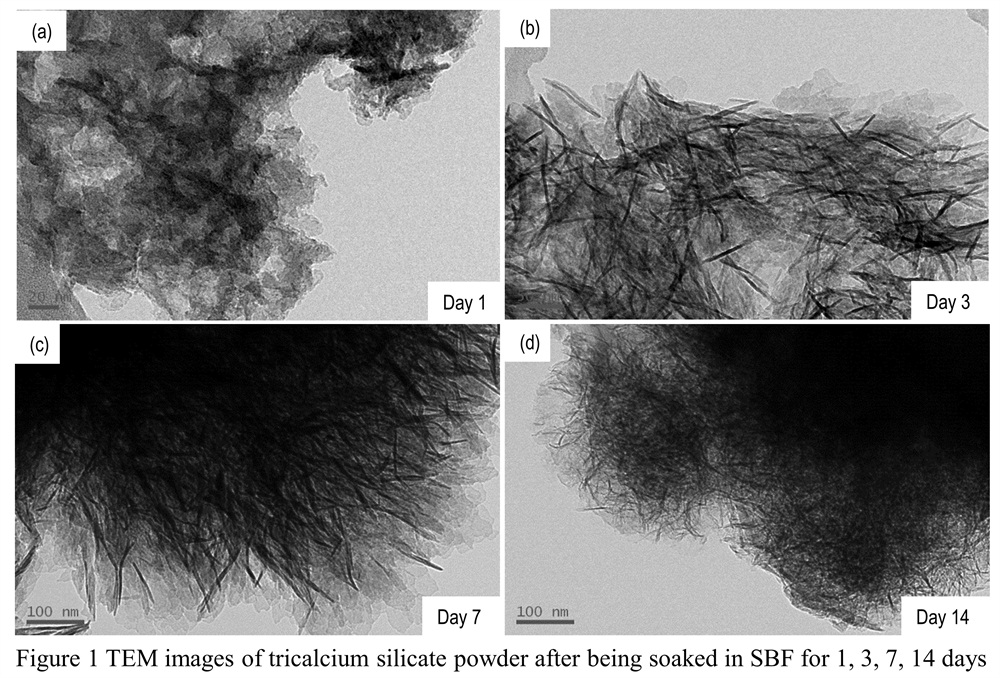
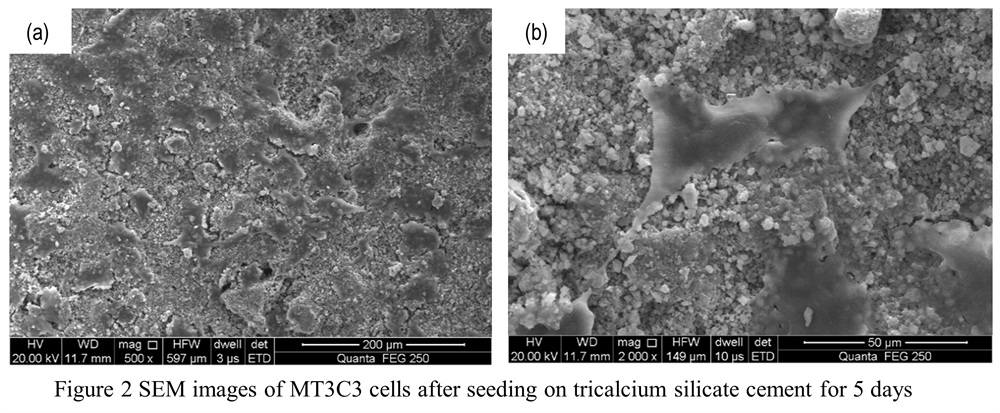
Results and Discussion: The XRD result shows that after calcination at 1200℃, there are three phases: Ca3SiO5, Ca2SiO4 and CaO. That’s because Ca3SiO5 is decomposed. After calcination at 1300 ℃, Ca3SiO5 was obtained with little CaO. CS powder can be synthesized successfully by Pechini method at 1400℃ and 3 h. The FTIR result confirms the formation of Ca3SiO5. The specific surface area of CS powder is 826.7 m2 /kg, which is higher than that synthesized by two step preicipitation method reported in ref[1]. After hydration,the results show that different L/P ratio had influence on the setting time and compressive strength of CS cement, the lower the L/P, and the shorter the setting time, the higher the compressive strength. The compressive strength was 22.9 MPa at L/P=0.6. After hydration, amorphous C-S-H inner product,fibrillar C-S-H outer product and plate-like portlandite (Ca(OH)2, PDF-78-0315) were formed in CSC. After being soaked in simulated body fluid, the SEM, XRD and EDX results showed that hydroxyapatite was formed on the surface of CS powder. TEM images of CS powder after being soaked in SBF for different time were shown in Fig.1. Si inons can be released from CSC, shown by ICP-AES result. The cell culture results showed that MT3C3 cells can grow well on the surface of CSC (Fig.2).
Conclusion: Pechini technique is an effective way to synthesize CS powder with shorter time and lower temperature. Tricalcium silicate cement that exhibits good bioactivity and biocompatibility, is a promising bone restorative material.
National Natural Science Foundation of China (No. 51504295)
References:
[1] Zhao W, Chang J. Two-step precipitation preparation and self-setting properties of tricalcium silicate. Materials Science and Engineering: C. 2008;28:289-93.