Islet-embedded endothelialized modules restore normoglycemia in diabetic SCID/Bg mice
-
1
University of Toronto, Institute of Biomaterials and Biomedical Engineering, Canada
-
2
University of Toronto, Department of Chemical Engineering and Applied Chemistry, Canada
The transplantation of pancreatic islets via the hepatic portal vein (Edmonton Protocol)[1] is a promising treatment for type 1 diabetics[1]. However, this therapy has limitations[2], two of which include: (1) the need for multiple donors for each transplant to achieve insulin independence[3] and (2) insulin independence that is not sustained[2]. Finding an alternative transplant site that is both minimally invasive and able to support a large transplant volume is necessary for preserving islet function. Although the subcutaneous site satisfies both of these criteria, the site is poorly vascularized. Modular tissue engineering is a “bottom-up” approach which uses collagen gel rods, mesenchymal stromal cells (MSC) and endothelial cells (EC) to form a subcutaneous vascularized bed and can be used to deliver pancreatic islets[4]. In this study, the subcutaneous transplantation of Wistar rat islets (750 IEQ, islet equivalents) embedded in endothelialized modules (mixed with EC modules with or without MSC) were sufficient to restore and maintain normoglycemia in strptozotocin induced diabetic SCID/Bg mice for up to 21 days (Fig. 1).
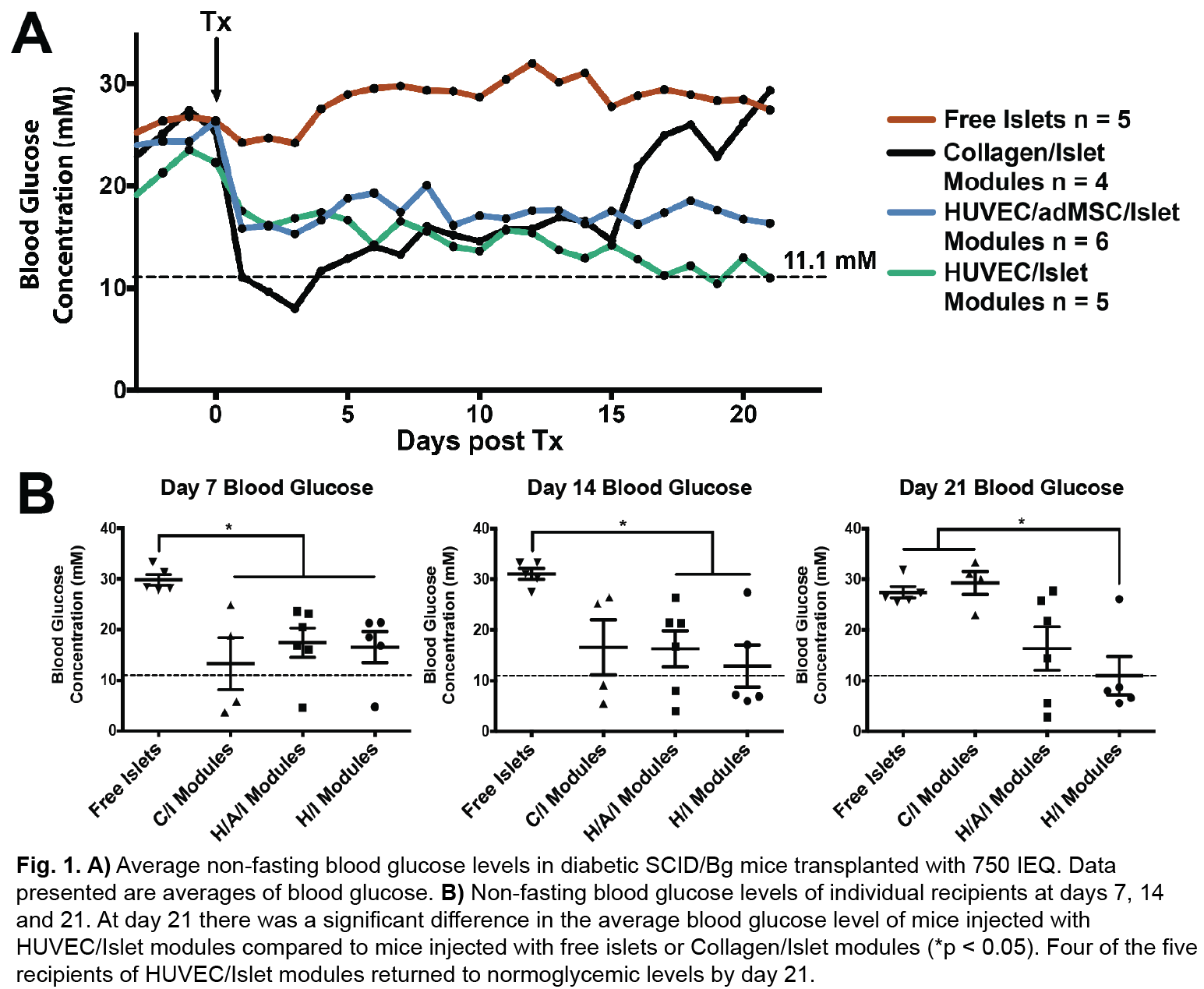
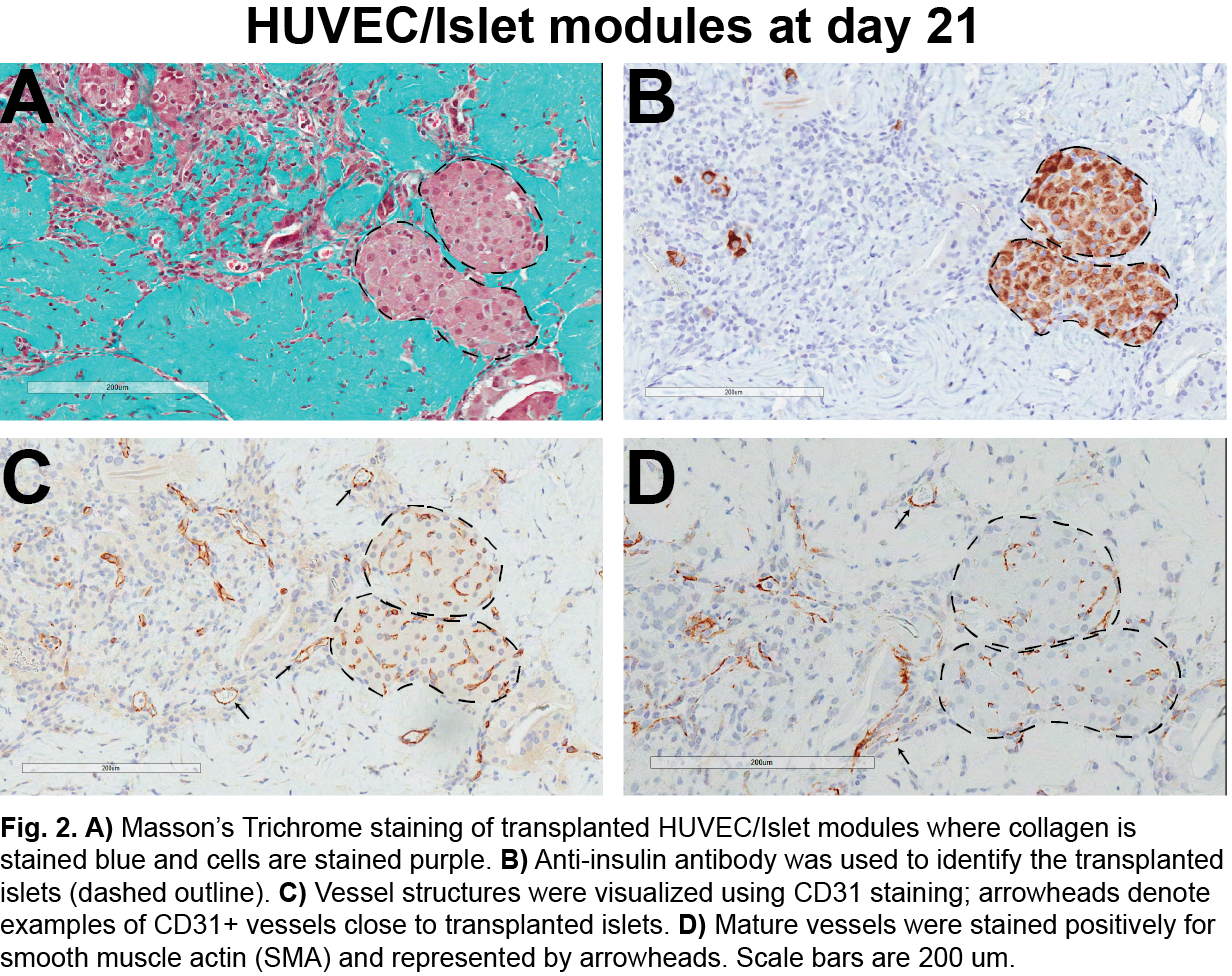
Free islets (no collagen or EC) had no effect. Islets embedded in collagen but without endothelial cells were able to return diabetic animals to normoglycemia, but insulin independence was not sustained (animals became hyperglycemic within 21 days). EC modules embedded with islets were well vascularized with mature host-derived vessels (Fig. 2).
While we have demonstrated that module-associated vasculature is sufficient to support islet function and viability over 21 days, it remains to reduce the number of islets required to achieve normoglycemia. To this end, we are using modules to pre-vascularize the subcutaneous site before islet transplantation in future studies.
Canadian Institute of Health Research; CIHR Training Program in Regenerative Medicine; Ontario Graduate Scholarships
References:
[1] Shapiro, A. M. J. et al. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med 343, 230–238 (2000).
[2] McCall, M. & James Shapiro, A. M. Update on Islet Transplantation. Cold Spring Harbor Perspectives in Medicine 2, a007823–a007823 (2012).
[3] Markmann, J. F., Deng, S., Huang, X. & Desai, N. M. Insulin independence following isolated islet transplantation and single islet infusions. Annals of … 237, 741–750 (2003).
[4] Butler, M. J. & Sefton, M. V. Cotransplantation of Adipose-Derived Mesenchymal Stromal Cells and Endothelial Cells in a Modular Construct Drives Vascularization in SCID/bg Mice. Tissue Engineering Part A 18, 1628–1641 (2012).
Keywords:
Regenerative Medicine,
biomedical application,
in vivo tissue engineering,
endothelialization
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Regenerative medicine: biomaterials for control of tissue induction
Citation:
Vlahos
A and
Sefton
MV
(2016). Islet-embedded endothelialized modules restore normoglycemia in diabetic SCID/Bg mice.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01372
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.