Introduction: Artificial blood contacting prostheses such as stents are widely used for clinical therapy. To date, however, complications such as thrombus and restenosis caused by insufficient biocompatibility of vascular stents continue to be the major limitation[1]. Besides, the performance of cardiovascular implants may also impeded by the foreign-body reaction that results in biofilm formation and restricts the function of cardiovascular implants[2]. Therefore, a novel kind of ultra-low-fouling and anticoagulant coating should be developed to prevent thrombosis and biofouling. Zwitterionic hydrogels which are generally viewed as a new and efficiency class of nonflouling materials were found to resist nonspecific protein adsorption in complex media in recent researches[3],[4]. However, the bioinert characteristic of such hydrogels always lead to poor cellular compatibility and thereby only limited attentions have been received. Focus on the cellular compatibility of zwitterionic hydrogels, in this study, vascular endothelial growth factors(VEGF) were incorporated into zwitterionic hydrogels to enhance mesenchymal stem cells adhesion and differentiation.
Materials and Methods: The poly(carboxybetaine methacrylate) (pCBMA) hydrogels were prepared by CBMA monomers and CBMA crosslinkers (CBMAX). The VEGF were then incorporated into this hydrogels via physical absorption, as shown in Fig.1a. After that, the functional zwitterionic hydrogels were immobilized to stainless steel surface with chemical modification (shown in Fig. 1b). The physicochemical properties of this zwitterionic hydrogels and the release rates of VEGF were characterized. The protein adsorption behavior and cellular compatibility of modified surfaces were evaluated by in vitro and in vivo (Fig. 1c).
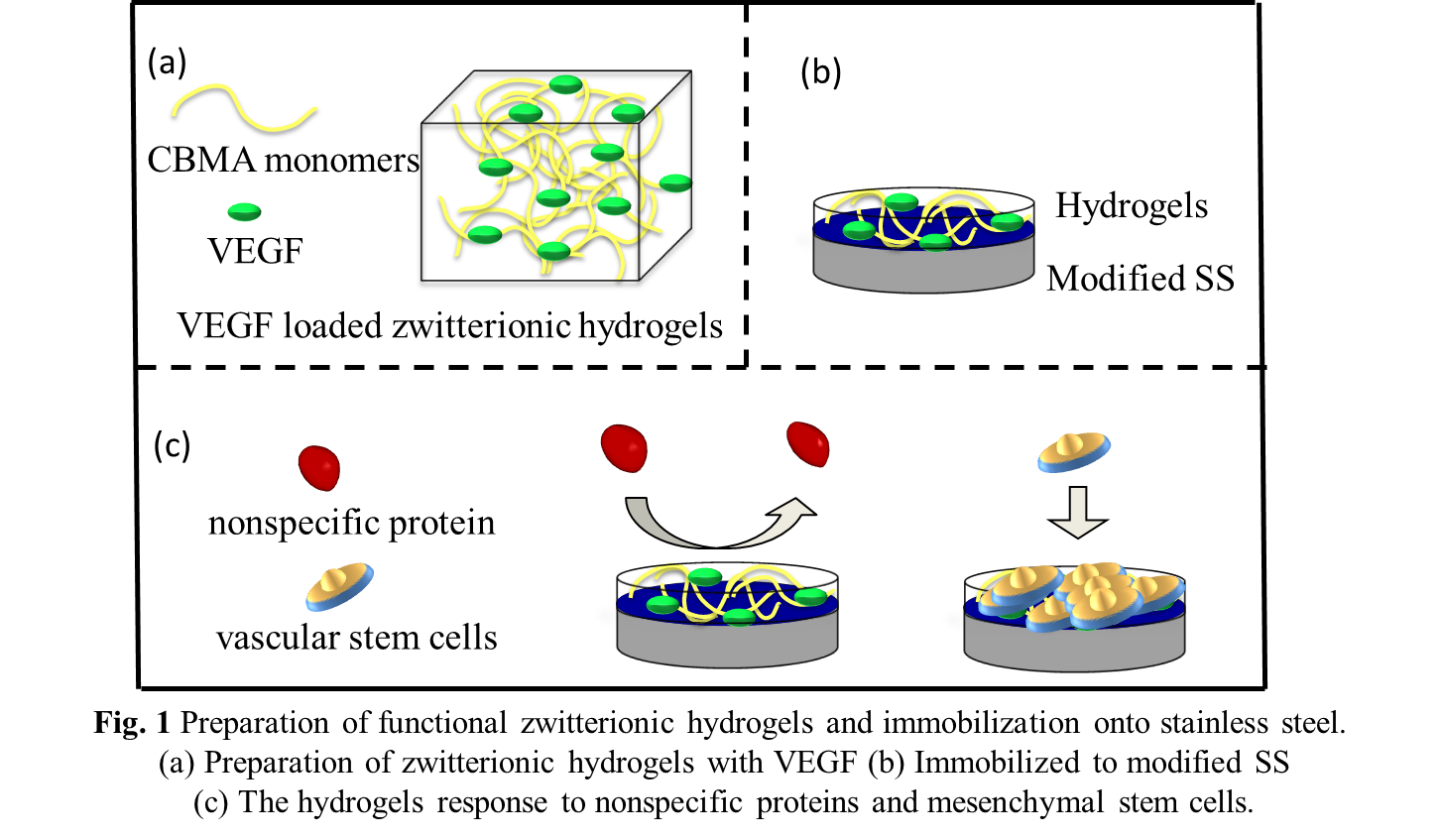
Results and Discussion: The results revealed that, the zwitterionic hydrogels with VEGF were successfully immobilized to stainless steel surface, and the VEGF deliver behavior was efficiently controlled. The dynamic evaluation in vitro results disclosed that the zwitterionic hydrogels possessed the antifouling property. The ex vivo investigation found that the zwitterionic hydrogels could capture the mesenchymal stem cells . In particular, the in vivo experiments displayed that the zwitterionic hydrogels could promote the in situ endothelialization.
Conclusions: All these results indicated that the zwitterionic hydrogels with VEGF possess the antifouling property, capture the stem cells, and improve the endothelium regeneration in situ. This study provides a promising approach applied zwitterionic hydrogels to cardiovascular implants surface modification.
This work was financially supported by the Key Basic Research Project (No. 2011CB606204#), and the National Natural Science Foundation of China (No.31170916#, No.31470921#).
References:
[1] Ren X.K., Teng Y.K., Guo J.T., Wang H.X., Li Q., Yang J., et al. Chem. Soc. Rev.44, 5680-5742, 2015.
[2] Langer R. .Adv. Mater. 21, 3235-3236, 2009.
[3] Zhang L., Cao Z.Q., Bai T., Carr L., Ella-Menye J.R., Irvin C., et al. Nature Biotechnology 31,553-556, 2013.
[4] Shao Q.,Jiang S.Y.. Adv. Mater. 27,15-26,2015.