Introduction: For human body tissue regeneration, different approaches, including scaffold-based[1], growth factor-based[2] and cell-based tissue engineering[3], are explored. In cell-based tissue engineering, developing appropriate vehicles for cell encapsulation and delivery is highly important. The process of cell encapsulation must be cell-friendly for achieving high cell viability. Moreover, for microspherical delivery vehicles, the diameter of cell-encapsulated microspheres is a critical factor. The diameter should not be too small or too large. Degradation properties of polymers for microspheres are another important factor for cell delivery. Coaxial electrospray can produce core-shell structured microspheres and hence was investigated in this study for the encapsulation and delivery of cells.
Materials and Methods: To determine suitable electrospray parameters, solid, cell-free microspheres were made first by electrospray using polymer solutions of sodium alginate (SA), poly(vinyl alcohol), gelatin and mixed solutions (1:1) of two polymers. Then, cell-free microspheres with core-shell structure were produced by coaxial electrospray where a polymer solution was fed into outer tube and PBS was fed into inner tube of the coaxial nozzle. After process optimization, PBS was replaced by aqueous cell suspensions of human umbilical vein endothelial cells (HUVECs) in coaxial electrospray for making cell-encapsulated microspheres. The morphology and structure of cell-free and cell-containing microspheres were studied. The viability of HUVECs was studied using live and dead assay. Triggered cell release was also investigated.
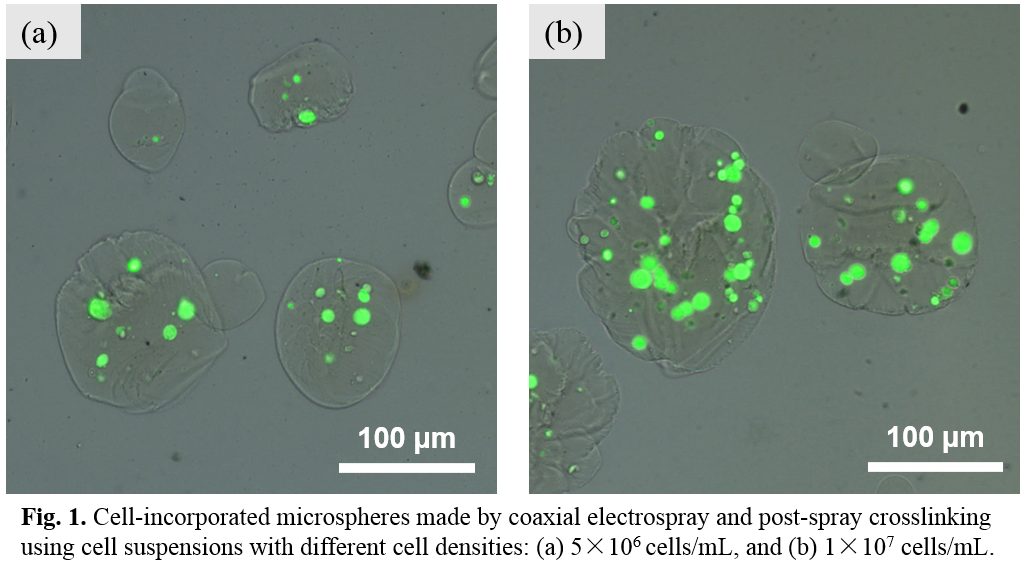
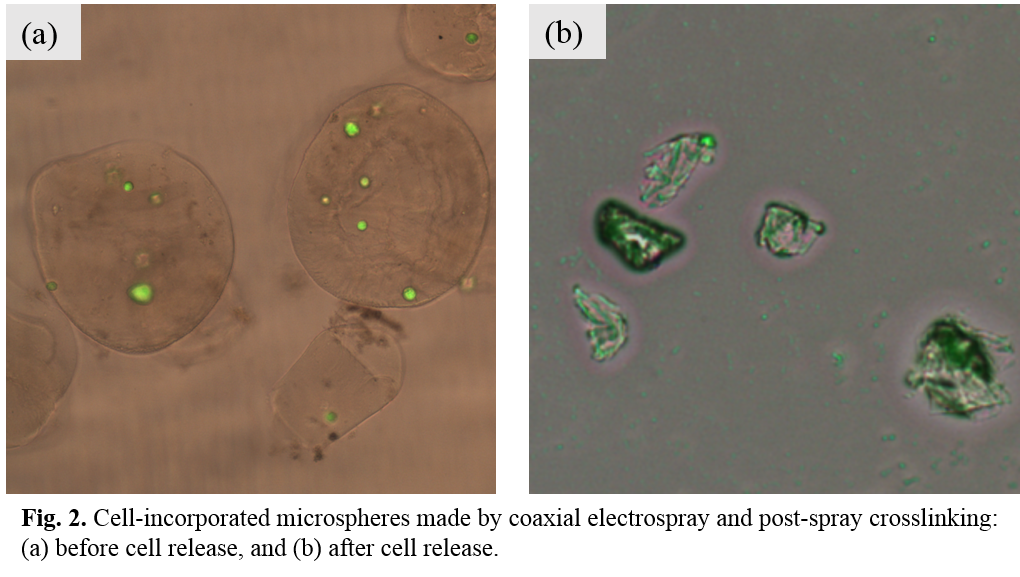
Results and Discussion: Electrospray experiments showed that relatively large diameter (~5 micron) and uniform microspheres could be produced using mixed polymer solution of SA and gelatin. Therefore, coaxial cell electrospray was conducted using HUVEC cell suspensions and SA/gelatin polymer solution or SA solution alone, and a post-spray cross-linking treatment was added for the polymer shell. After further explorations, electrospray parameters (applied voltage, feeding rate ratio between the outer tube and inner tube, etc.) were fixed for fabricating cell-encapsulated microspheres. HUVECs with low cell density (5×106 cells/mL) and high cell density (1×107 cells/mL) were used for encapsulation. Fig.1 shows cell-encapsulated microspheres made by coaxial electrospray and post-spray crosslinking using different cell densities. Microspheres with regular spherical shape could be produced for both low cell density and high cell density. A higher cell loading efficiency was attained for microspheres using a higher cell density. Triggered cell release was achieved by treating cell-encapsulated microspheres with a 0.05M sodium citrate solution. The polymer shell of microspheres was dissolved gradually and after 10 minutes, polymer shell disappeared and cells were totally released (Fig.2).
Conclusion: Coaxial electrospray proved to be a useful and adaptable technique for making cell delivery vehicles. With process optimization, sufficient cells could be encapsulated in core-shell structured microspheres via coaxial electrospray. Cells contained in the core of microspheres were well protected and showed high viability after the electrospray process. Triggered release could be realized for delivering live cells, enabling cell-based therapies.
This work was supported by Hong Kong Research Grants Council through a GRF Grant (HKU 717713E).
References:
[1] C. Wang, M. Wang, Electrospun multifunctional tissue engineering scaffolds, Front. Mater. Sci., 2014, 8, 3.
[2] J. E. Babensee, L. V. McIntire, et al., Growth factor delivery for tissue engineering, Pharm. Res., 2000, 17, 497.
[3] W. L. Fodor, Tissue engineering and cell based therapies, from the bench to the clinic: the potential to replace, repair and regenerate, Reprod. Biol. Endocrinol., 2003, 1, 102.