Introduction: Core cross-linked star (CCS) polymers represent an intriguing platform in the application of drug delivery. CCS polymers as drug carriers are more robust to environmental variations, compared with the self-assembled micelles or vesicles, which tend to disassemble below their critical association concentration or under external stimuli, followed by an undesired burst release of drugs[1],[2]. Also, the ease of designing the size or functionality of arms and cores separately makes CCS polymers more attractive than other types of unimolecular containers[3].
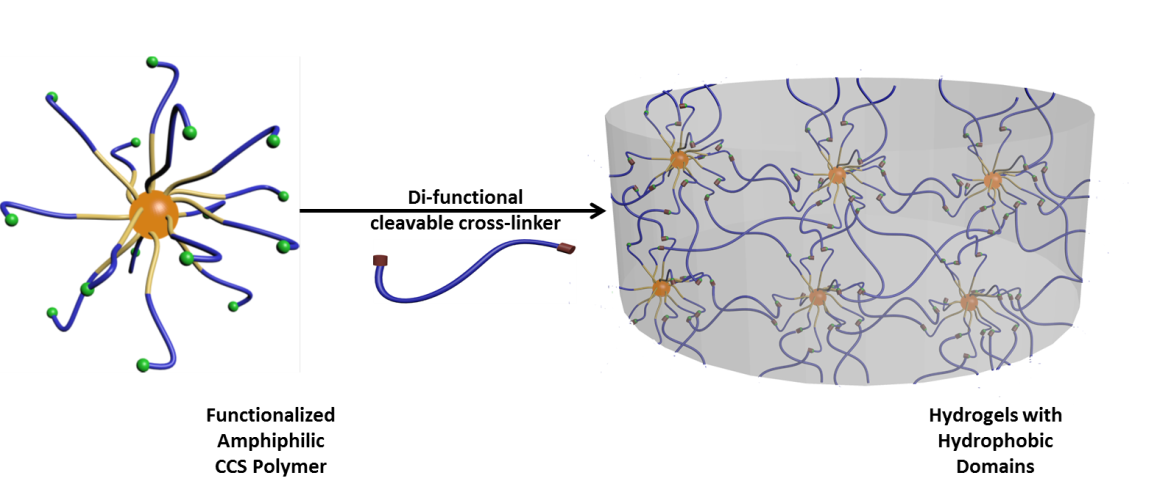
Like many other molecular carriers, however, the limited circulation period of CCS polymers in body would decrease the duration of drug action. In order to overcome this constraint, CCS polymers can be built into hydrogel to form a stable drug-delivery implant.
Materials and Methods: In this work, CCS polymers were obtained by using ring-opening polymerization (ROP) in two steps, with firstly poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) copolymers being formed as macroinitiators (MIs) and then these MIs being cross linked by [4, 4'-bioxepane]-7, 7'-dione (BOD) (Fig1)[4]. Various characterization tools, such as NMR, GPC, DLS, TEM, UV-Vis spectroscopy, flow cytometry and CLSM, have been adopted to investigate the structures, drug loading and release properties (pirarubicin and doxorubicin used as model hydrophobic drugs), cytotoxicity and cellular uptake of the resulting CCS polymers.
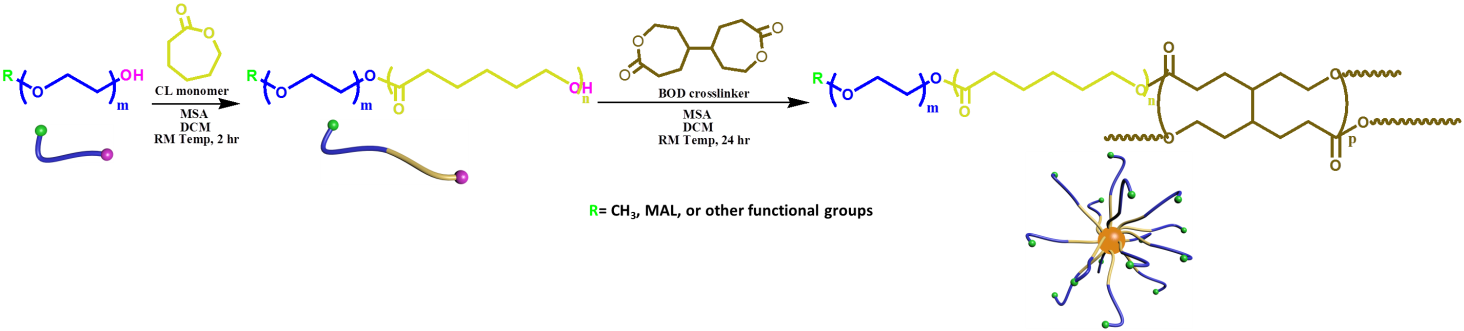
Furthermore, the functionalized PEG arm was used to introduce the reactive end groups in the outer layer of the CCS, allowing for the formation of hydrogels in the presence of certain cross-linkers (Fig2).
Results and Discussion: A range of CCS polymers were made with different hydrophilic/hydrophobic balance by tuning the ratio of PEG to CL and BOD blocks. These star polymers are soluble in both organic solvents and water and exist in a unimolecular state, as characterized by DLS and TEM, which provides the opportunity to easily stabilise hydrophobic drugs in aqueous environment without the need for lengthy encapsulation techniques.
Their drug loading capacity is heavily influenced by the hydrophobicity and core size. The in vitro drug release study illustrated high stability of CCS-drug complex at neutral pH and a faster release profile under acidic conditions due to the degradation of pH-sensitive PCL segments.
The CCS polymers also demonstrated very low toxicity, with cell viability remaining above 80% even up to high polymer concentrations. CLSM and flow cytometry analyses indicated highly efficient cellular uptake.
The hydrogels based on the PEG-PCL-BOD CCS polymers feature the uniform distribution of hydrophobic domains as the hydrophobic drug depots. The swelling, mechanical and drug loading properties of the hydrogels are largely determined by the variations in hydrophobic content of the embedded CCS polymers.
Conclusions:
- A library of well-defined amphiphilic PEG-PCL-BOD CCS polymers was prepared through facile ROP.
- They have large loading capacities for hydrophobic drugs, with the CCS-drug complex displaying high stability at neutral pH and a faster release under acidic condition.
- The unimolecular drug containers are bio-compatible and can be internalised by cells with high efficiency.
- The functionalized CCS polymers could become key building blocks of the hydrogels with hydrophobic domains as drug-delivery implants for cancer therapy.
The authors acknowledge the Australian Research Council under the Future and Super Science Fellowship Schemes (FT110100411, G.G.Q.; FS110200025, G.G.Q. & K.L.).; D.G. thanks The University of Melbourne for the provision of MIRS.
References:
[1] H. Wei, X. Zhang, C. Cheng, S. X. Cheng and R. X. Zhuo, Biomaterials, 2007, 28, 99-107.
[2] S. Peng, K. Wang, D. S. Guo and Y. Liu, Soft Matter, 2014, 11, 290-296.
[3] J. T. Wiltshire and G. G. Qiao, Aust J Chem, 2007, 60, 699-705.
[4] D. Gu, K. Ladewig, M. Klimak, D. Haylock, K. M. McLean, A. J. O'Connor and G. G. Qiao, Polymer Chemistry, 2015, 6, 6475-6487.