Introduction: Poly(trimethylene carbonate) (PTMC) attratcs increasing attention as a degradable polymeric platform for biomedical applications, due to the softness and its unique degradation behavior unlike biodegradable polyesters.[1] In addition, PTMC analogs with a functional side chain (PMTC) now afford many functionalized implantable biomaterials.[2] Recent progress in organocatalysis allows controlled polymerization of MTCs, monomers of PMTC, enabling formation of complex architecture and multi-functionalization.[3]
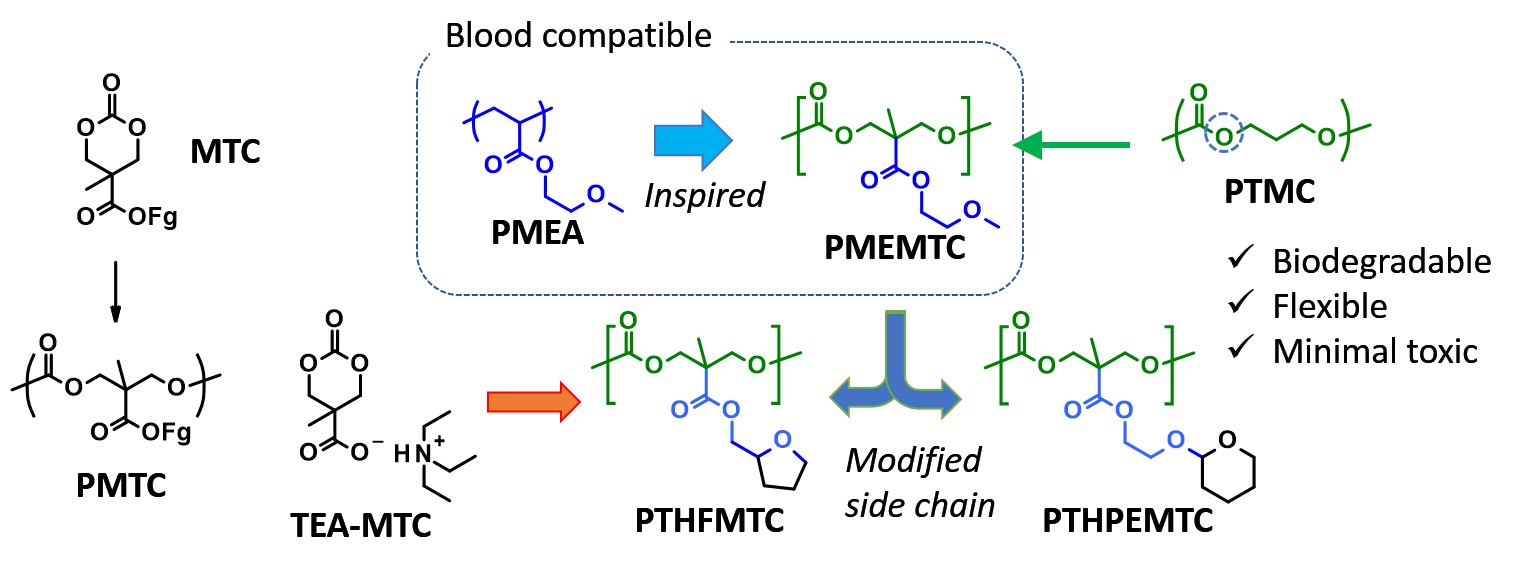
One of the critical features as biomaterials used in the body involves hemocompatibility. So far, few biodegradable polymers have presented sufficient blood compatibility. Then, we focused on a blood compatible polymer, poly(2-methoxyethyl acrylate) (PMEA). According to Tanaka et al., the ether side chain plays a significant role in hydration and formation of ‘intermediate water’ that is recognized to contribute to suppression of protein and platelet adsorption.[4] Thus, we have produced poly(2-methoxyethyl 2-methyltrimethylene carbonate carboxylate) (PMEMTC), demonstrating decent hemocompatibility and enzymatic degradability. However, as for the vascular regeneration application, adhesion of endothelial cells (ECs) is also important. Poly(tetrahydrofurfuryl acrylate) (PTHFA) is another blood compatible polymer and effective in ECs attachment and proliferation. Thus, we designed new analogs of PMEMTC with cyclic ether moiety at the side chain to install both hemocompatibility and EC adhesion property. Furthermore, we also developed a new facile synthetic route to MTC-type monomers through an ammonium salt intermediate TEA-MTC (Figure 1).
Results and Discussion: PTHFMTC and PTHPEMTC were successfully prepared as a consequence of the relatively controlled ROP, allowing for Mn and molar mass distribution, 11,000 (1.12) and 9,400 (1.14), respectively. All carbonate polymers used in this study were hydrophobic. Interestingly, contact angles were 61° for PTHFMTC and 58° for PTHPEMTC, which are comparable to that of PMEMTC, despite more hydrocarbons installed.
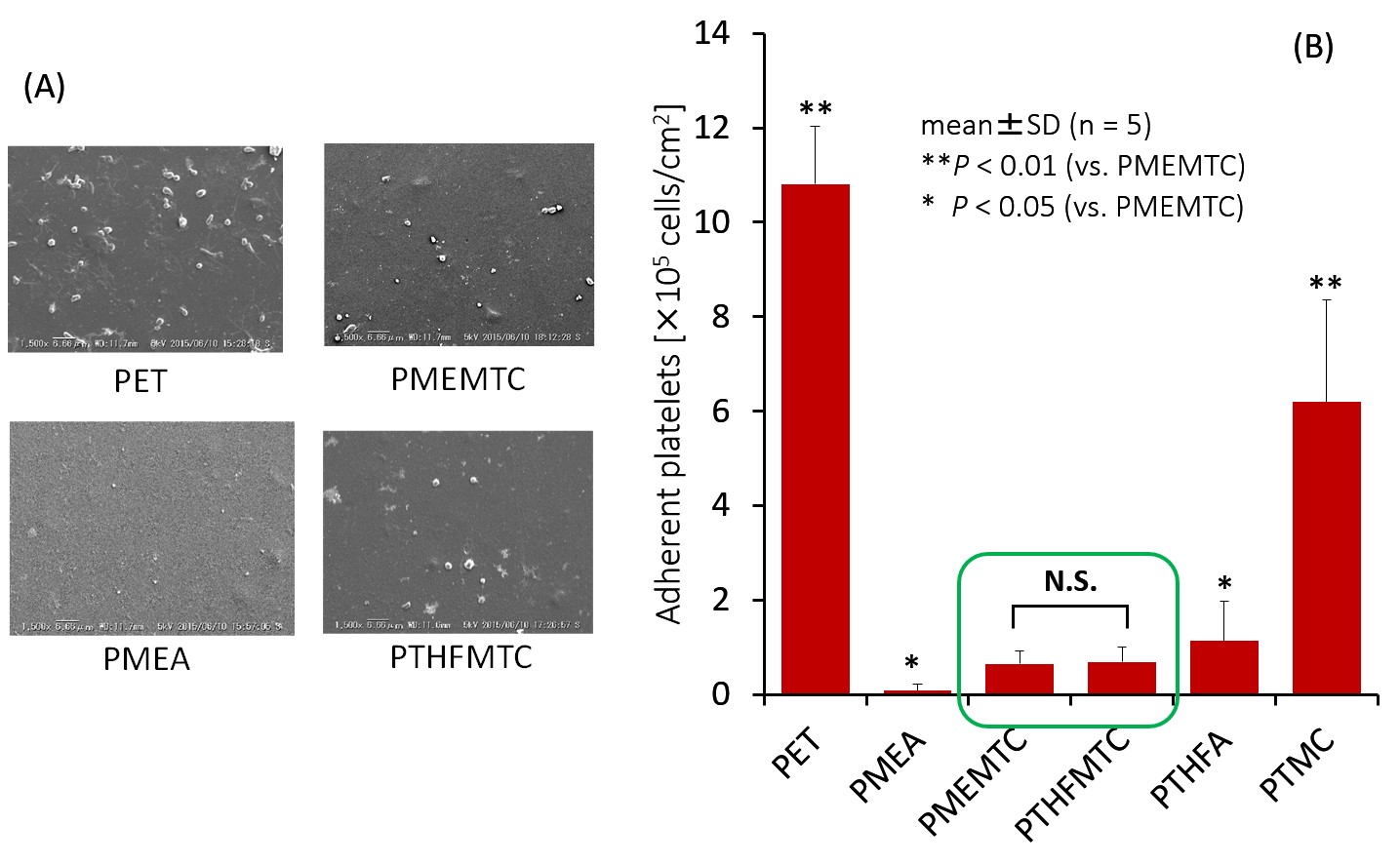
Figure 2 represents the comparison of adherent human platelets on the polymer surfaces after treatment at 37°C for 1 h. A few platelets adhered on PTHFMTC and PMEMTC. The numbers of adherent platelets are slightly higher than that on PMEA, but significantly lower than that on PTMC.
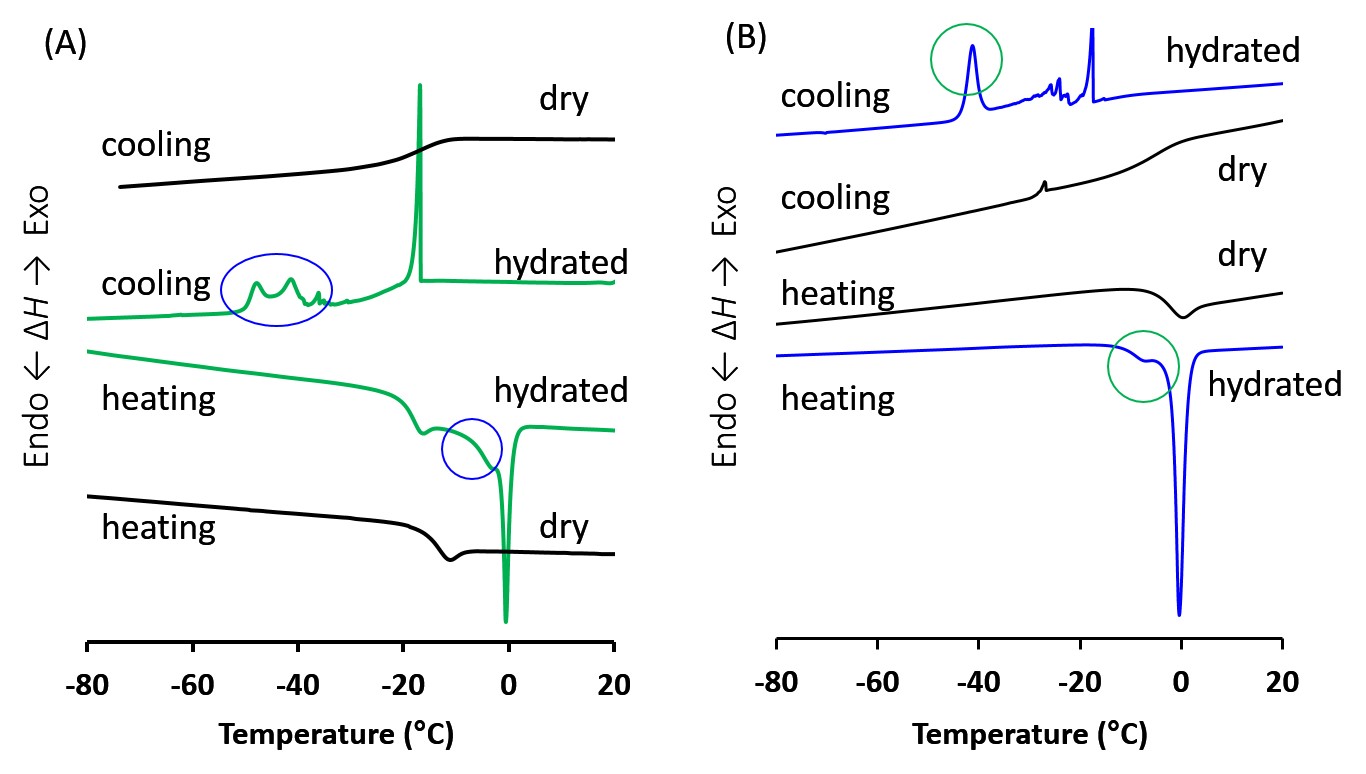
Then, we investigated water structure of hydrated water bound by polymer structure. On the cooling DSC chart of the hydrated polymers, crystallization exotherms were observed around -40°C for both PMEMTC and PTHFMTC (Figure 3). These crystalized water peaks should not be asigned as free or bulk water, exhibiting the exotherm above -20°C, according to our recent report.[5] Thus, we currently believe that this type of water also is attributed to suppression of platelet adhesion. Further studies are ongoing. The results of EC adhesion test will also be discussed. Preliminarily, human umbilical vein endothelial cells (HUVECs) were found to reasonably adhere on PMEMTC at 1 and 3 days after incubation.
Conclusion: These (potentially) biodegradable polycarbonates with ether side chain can be applicable for both a coating of blood contacting devices and a candidate scaffold for vascular regeneration.
References:
[1] K. Fukushima, Biomater. Sci., in press
[2] D. P. Sanders et al., J. Am. Chem. Soc., 2010, 132, 14724–14726.
[3] A. P. Dove, ACS Macro Lett., 2012, 1, 1409-1412.
[4] M. Tanaka et al., Polym. J. 2015, 47, 114-121.
[5] K. Fukushima et al., Polym. J., 2015, 47, 469-473