Transplantation of encapsulated pancreatic islets is a treatment for type 1 diabetes that has the potential to reduce or eliminate the need for exogenous insulin and chronic immunosuppression. The most commonly used biomaterial for this purpose is alginate, a polymer derived from brown algae. A major obstacle to islet transplantation is the lack of adequate islet oxygenation, leading to impaired glucose-responsive insulin secretion[1] and central necrosis[2]. Numerous strategies have been proposed to oxygenate islets in order to prolong the longevity and efficacy of the transplants, but few have been successfully established so far in animal models. The objective of this work was to develop a vascularized islet transplantation device using 3D printing methods and islet immobilization in hydrogels.
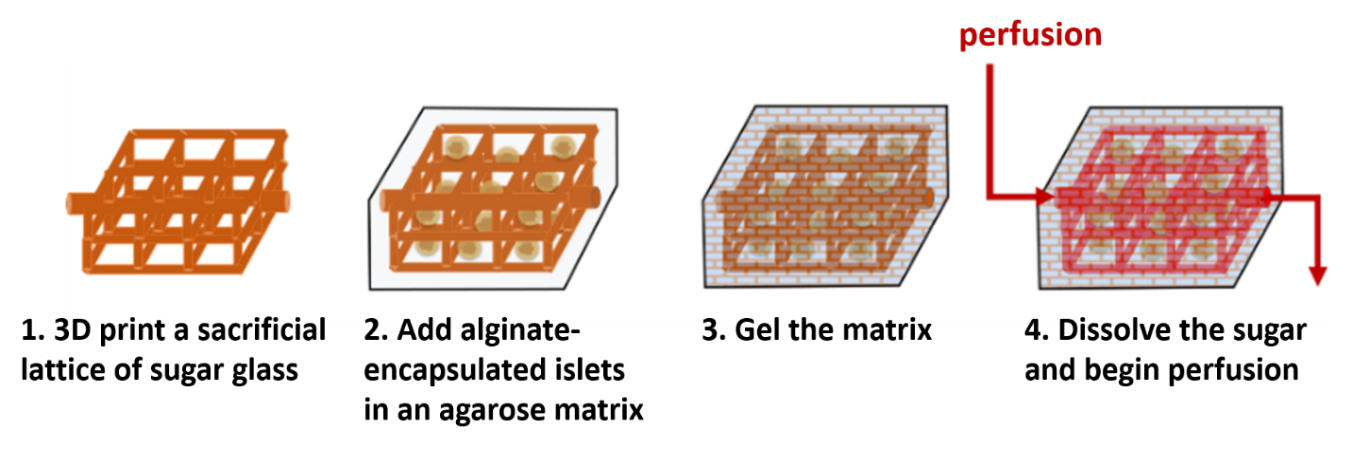
Figure 1: Steps for fabricating a vascularized islet transplantation device.
Artificial vasculature is 3D printed using a sugar glass mixture (Figure 1), composed of glucose, sucrose and reverse osmosis water. Printing is done with a modified 3D printer, using a heated glass syringe as a sugar reservoir, and extruding through a 1 mm-diameter custom-made nozzle. The syringe and nozzle temperature, as well as the extrusion rate, can be independently controlled. Once printed, the sugar networks are coated with poly(lactide-co-glycolide) and embedded in a 1.5% agarose matrix. Dispersed throughout this matrix are islets encapsulated in alginate via an emulsion and internal gelation process[3]. After agarose gelation by mild cooling, the entire construct is submerged in aqueous medium to dissolve the sugar glass, leaving hollow perfusable channels that mimic vasculature.
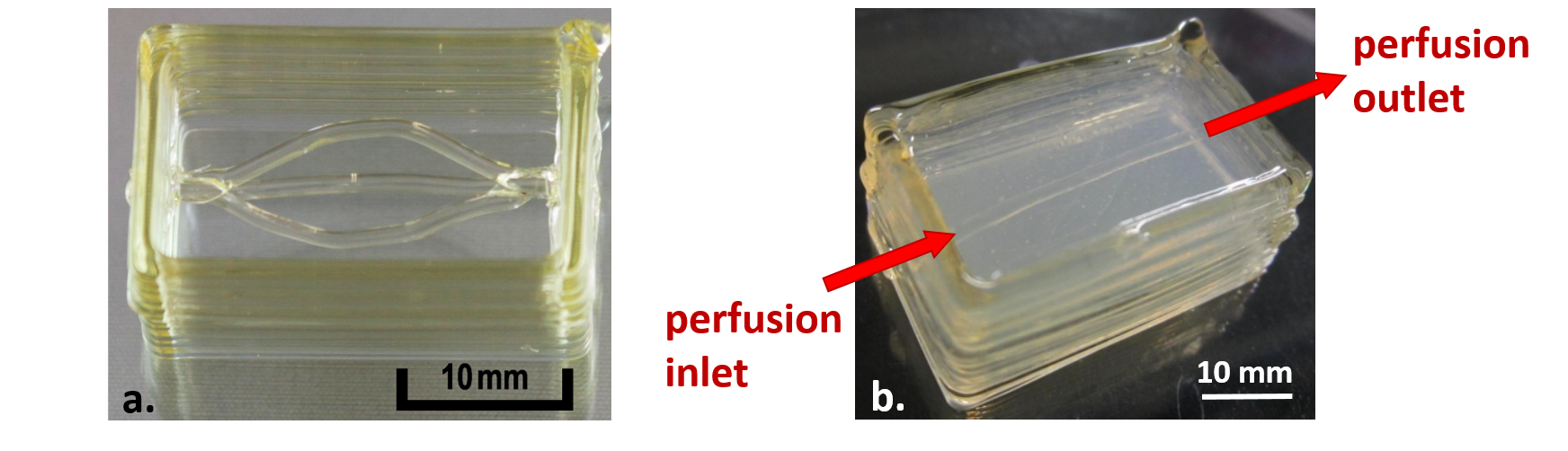
Figure 2: 3D printed sugar glass constructs featuring a) a branched channel and b) a single channel embedded in a 1.5% agarose hydrogel block.
Initially, the sugar glass composition proposed by Miller et al[4]. was used; however, this composition led to highly variable sugar constructs of poor durability. Miller’s recipe was adapted to generate a homogeneous sugar glass mixture, and stable sugar prints with channel diameters ranging from 0.5–2 mm were obtained in a reproducible and controlled manner (Figure 2a). Single-channel sugar prints were embedded in 1.5% agarose (Figure 2b), and the sugar was dissolved and evacuated through submersion in pH 7.4 HEPES buffer. Blue dextran (2000 kDa) solution was perfused through the single channel without visible diffusion into the hydrogel. The next step is to repeat this perfusion with different dextran sizes to determine the size-exclusion properties of the hydrogel. Encapsulated islets will be introduced to study cell viability, growth and insulin secretion within the 3D construct. Long-term goals include printing more complex lattice structures of varying densities and comparing the effects on oxygen diffusion and islet behaviour. This work constitutes a first step towards improving the success of islet transplantation, as well as developing a means for studying and enhancing oxygen diffusion in other 3D cell culture systems.
McGill University; Juvenile Diabetes Research Foundation (JDRF); Diabète Québec; Montréal Diabetes Research Centre (MDRC); Natural Sciences and Engineering Research Council of Canada (NSERC); Fonds de recherche du Québec - Nature et technologies (FRQNT); Centre québécois sur les matériaux fonctionnels (CQMF); Le Réseau de thérapie cellulaire et tissulaire du FRQS (ThéCell); PROTÉO Network
References:
[1] Dionne KE et al. (1993) Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes, 42(1):12-21.
[2] Olsson R and PO Carlsson. (2006) Oxygenation of cultured pancreatic islets. Adv Exp Med Biol, 578:263-268.
[3] Hoesli CA et al. (2011) Pancreatic cell immobilization in alginate beads produced by emulsion and internal gelation. Biotechnol Bioeng, 108(2):424-434.
[4] Miller JS et al. (2012) Rapid casting of patterned vascular networks for perfusable engineered three dimensional tissues. Nat Mater, 11(9):768-774.