Introduction: Hemostasis of unexpected massive bleeding frequently occurred in surgical procedures that causes early death by trauma or infectious complications is a challenging issue. Although hemostatic materials are being extensively developed, arresting perioperative bleeding of patients with no or delayed capability of hemostasis such as hemophilia, anemia, diabetes, and thrombocytopenia is a difficult task. Herein, we reported a novel design principle of hemostats for delayed hemostasis or coagulopathy by instant formation of adhesive sealant membrane. We unexpectedly observed that chitosan-catechol formed porous membranes in blood plasma at the early stage, and then blood protein barriers were formed by interactions of chitosan-catechol and blood proteins. Thromboelastography of patient cases also implied that successful hemostasis caused by complexation of chitosan-catechol and blood proteins. Our results demonstrate that these chitosan-catechol hemostatic materials robustly arrest the bleeding for all patients regardless of their medical history and safely use in surgical procedures.
Materials and Methods: Chitosan-catechol was synthesized using standard EDC chemistry. Briefly, chitosan (3 g, 19.48 mmol) was dissolved in pH 5 HCl solutions (100 mL). HCA (2.37 g, 15.58 mmol) and EDC (2.02 g, 10.54 mmol) were dissolved in deionized and distilled water (DDW, 25 mL), respectively, and then both were slowly added to the chitosan solution. Ethanol (50 mL) was used as a co-solvent to prevent the possible precipitations during EDC coupling reaction. After 12 hrs, the product was purified by membrane dialysis (MWCO: 12,000-14,000, SpectraPor, USA) against pH 4.0 NaCl solutions for 2 days and DDW for 4 h, and then freeze-dried.
Results and Discussions: We found that chitosan-catechol formed porous membranes instantly upon adding the solution to blood plasma. Briefly, we obtained blood plasma by the centrifugation, and then chitosan-catechol solutions (0.5 wt%) were dropped into the blood plasma (Figure 1A). Chitosan-catechol immediately formed porous membrane (Figures 1B, left).
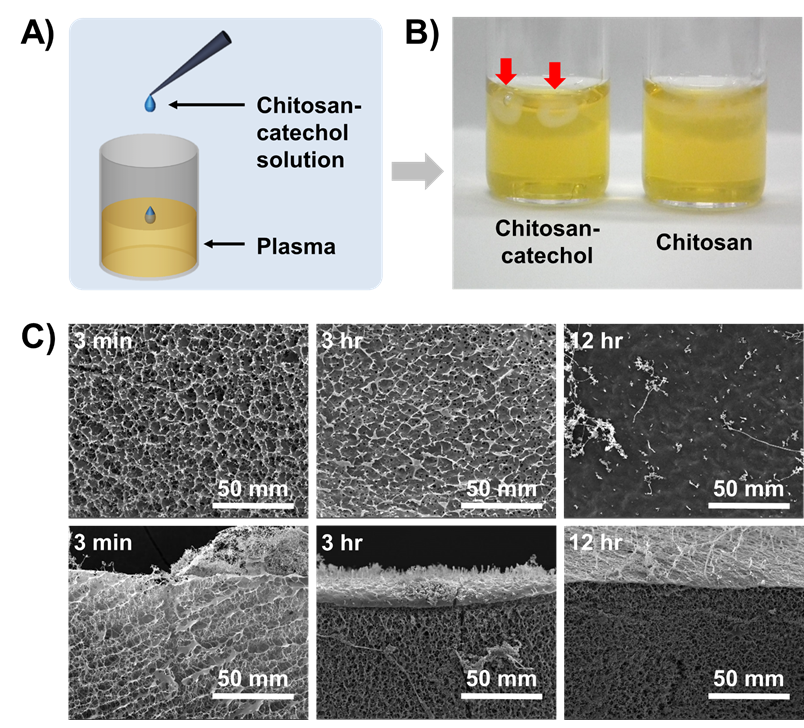
Fig. 1. Formation of chitosan-catechol/blood plasma protein sealant membranes.
In contrast, we could not observe the membrane formation when using chemically unmodified chitosan (Figure 1B, right) demonstrating that the conjugation of catechol showed clearly different behaviors at a molecular level. From these results, we designed an experiment to reveal the interactions between chitosan-catechols and biomacromolecules in human bloods kinetically. To monitor the morphological changes of bead formation, we prepared chitosan-catechol beads in blood plasma at a pre-determined time interval. As shown in Figure 1C, micro-porous membranes by chitosan-catechol/blood plasma complexation were obtained. The membrances were formed within 3 min. In case of chitosan-catechol beads after 12 hr incubations, a stiff film was formed at outside by further protein complexation. Unlike the mechanism of hemostatic action of fibrin-based glues, chitosan-catechol rapidly adhered to hemorrhage sites and immediately arrested the bleeding by forming blood protein barriers..
Conclusion: The hemostatic material, chitosan-catechol, is expected to provide effective hemostasis for preventing bleedings for patients such as hemophila, anemia, thrombocytopenia, liver transplantation and etc.