Introduction: Abdominal aortic aneurysm (AAA) is a multifactorial degenerative disease with infiltration of inflammatory cells, degradation of the extracellular matrix (ECM), apoptosis of smooth muscle cells and thinning of the tunica media[1]. AAA is thought to develop as a consequence of unbalanced activity of matrix metalloproteinases (MMP), a family of proteolytic enzymes targeting various components of ECM. In the attempt to stabilize in an early stage aneurysmal disease, thus preventing further expansion and surgery treatment, in the last years, many MMP’s inhibitors have been developed. The most efficient method to control MMPs’ expression is the use of the RNA interference (RNAi) technique. RNAi inhibits gene expression by causing the destruction of mRNA encoding the target protein[2]. MMP2 and MMP9 are interesting targets for shRNA-based on molecular therapy. Here, we evaluated the approach of lentiviral vector (LV) directed gene transfer of shRNA for aneurysm molecular therapy.
Materials and Methods: Samples of AAA were surgically recovered; control aortic tissue was obtained at autopsy. Human aorta wall structural modifications were investigated with haematoxylin and eosin (H&E) and Weigert staining for elastic tissue. For immunofluorescence, histological sections were processed with primary antibodies (anti-MMP2, -MMP9 and -Elastin) followed by a secondary antibody-FITC or TRITC incubation. For immunohistochemistry, the reaction was detected using the avidin–biotin method. Gelatin zymography was performed for detecting MMP2 and MMP9 activity. A third generation LVs containing the specific shRNA for silencing of MMP2 and MMP9 were designed. LV were produced and tittered before silencing experiments were performed on smooth muscle cell and blood monocyte cell lines.
Results: H&E revealed numerous inflammatory cells whereas immuno-histological staining showed that elastic laminae was not maintained in aneurysm wall. As shown in Figure 1, there was no significant difference in MMP2 expression between control and aneurysm samples, while a significant difference for MMP9 expression has been observed, whereMMP9 resulted several folds up-regulated in the aneurysm wall. Data have been also confirmed by zymography assay. For MMP2 and MMP9 silencing experiments, MMPs expression was induced in smooth muscle and monocyte cells, respectively. A really high efficiency of transduction was obtained using LV-shRNA for MMP2 and 9 on treated cells. MMPs expression on both cell lines treated with LV-shRNA-MMP2 or LV-shRNA-MMP9 showed a significant inhibition of MMP in transduced cells with respect to control treated with a LV-shRNA.
Conclusion: Data obtained on human samples confirmed the key role of MMP9 in aneurysm development. LV transduction is an efficient method to obtain gene silencing in transduced cells. The use of this technique can prospectively represent an innovative approach in the treatment of aneurysms by molecular therapy in situ.
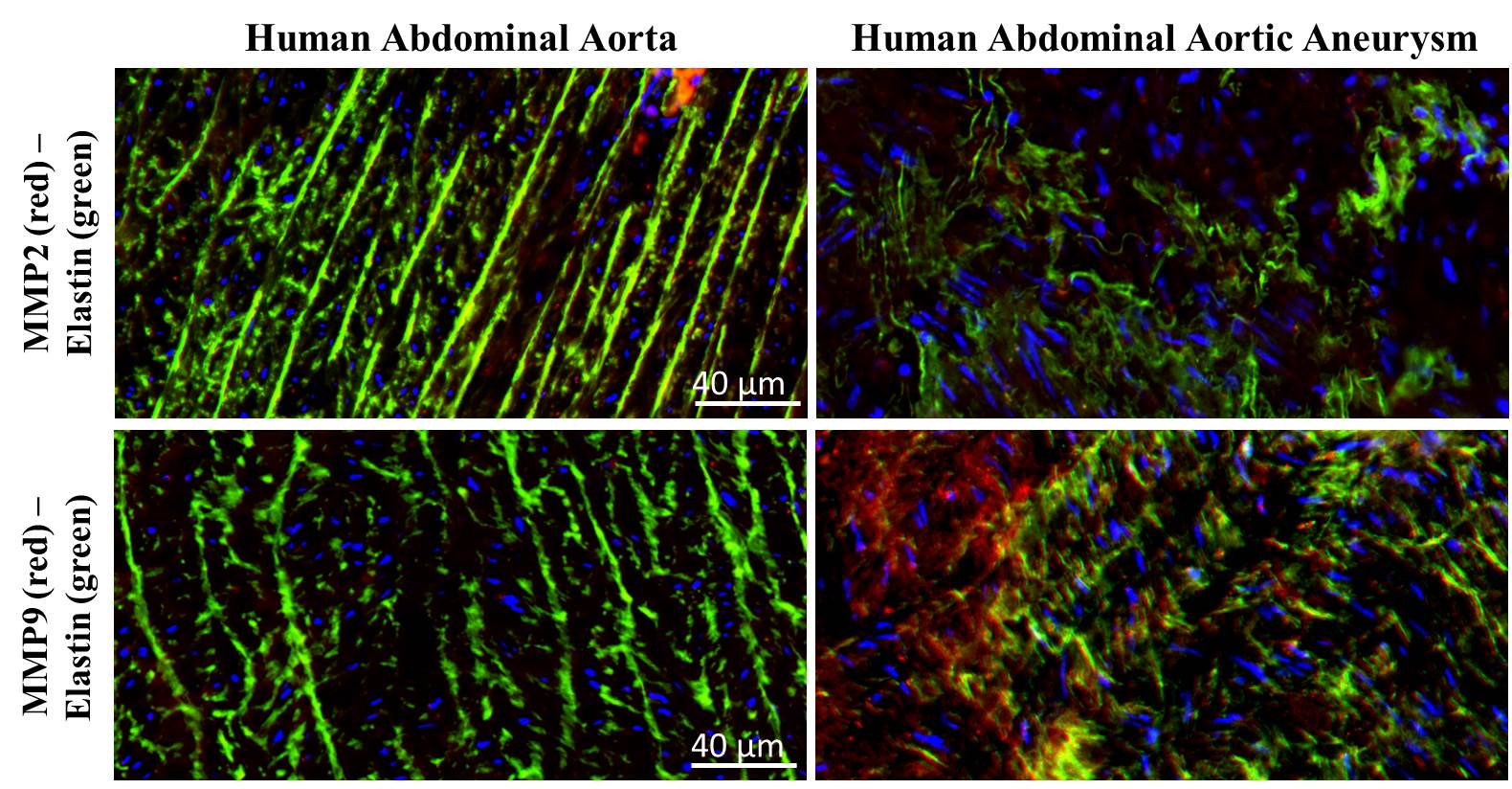
Figure1. Immunofluorescence staining on human control aorta and abdominal aneurysm for MMP2 and MMP9 (red) and Elastin (green). DAPI (blue) is used for nuclear staining.
References:
[1] Zong-Zhuang Li and Qiu-Yan Dai. Pathogenesis of Abdominal Aortic Aneurysms: Role of Nicotine and Nicotinic Acetylcholine Receptors. Mediators Inflamm. 2012; 2012: 103120.
[2] McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737-747.