Introduction: Vinyl polymers have been the focus of intensive research over the past few decades, leading to the development of controlled radical polymerization (CRP) techniques, such as nitroxide-mediated polymerization (NMP)[1], atom-transfer radical polymerization (ATRP)[2] and reversible addition-fragmentation chain transfer polymerization (RAFT)[3]. CRP techniques enable the synthesis of materials with a broad diversity of architectures, compositions and functionalities. Yet, the resistance of vinyl backbones to degradation may limit their application in major fields of research, including (nano)medicine and environmental protection. Therefore, the development of synthetic strategies to enable complete or partial degradation of vinyl polymers is key to new opportunities for the application of these materials[4]. To successfully synthesize (bio)degradable controlled vinyl polymers, we investigated the combination of NMP of methacrylate derivatives with radical ring-opening polymerization (rROP) of various cyclic ketene acetals (CKAs) (see Figure 1), from copolymerization to degradability and cytotoxicity.
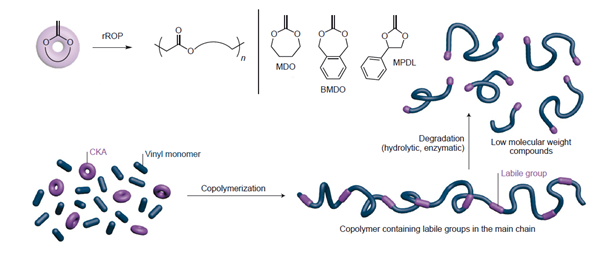
Figure 1. Synthesis of (bio)degradable vinyl polymer comprising multiple ester functions, by radical copolymerization of vinyl monomer and cyclic ketene acetal (CKA) (from ref 4).
Materials and Methods: Three CKAs, namely 2-methylene-1,3-dioxepane (MDO), 5,6-benzo-2-methylene-1,3-dioxepane (BMDO) and 2-methylene-4-phenyl-1,3-dioxolane (MPDL), were synthesized and copolymerized with methyl methacrylate (MMA) and oligo(ethylene glycol) methyl ether methacrylate (OEGMA). Polymerization livingness was assessed by 31P NMR and confirmed by the synthesis of a library of block copolymers. Copolymer degradation was investigated at basic and neutral pH, and their cytotoxicity was assessed on three different cell lines (NIH/3T3, HUVEC and J774 cells) by MTT assay at 2 different concentrations.
Results and Discussion: Among the three CKAs tested, MPDL showed a unique ability to copolymerize with methacrylate derivatives by NMP, with dispersities as low as 1,25 and living fraction up to 75%[5], which further allowed block copolymer synthesis[6]. Hydrolysis showed predictable molar mass reductions, from 25% to 95%, as a function of the amount of MPDL in the monomer feed. By further investigating structures and properties of MPDL-containing copolymers, we demonstrated that the styrene-like ring-opened structure of MPDL allows it to act as a controlling comonomer while inserting degradable units[7]. Finally, the innocuousness of these copolymers and their degradation products was confirmed in vitro, with cell viability ranging from 75 to 100%.
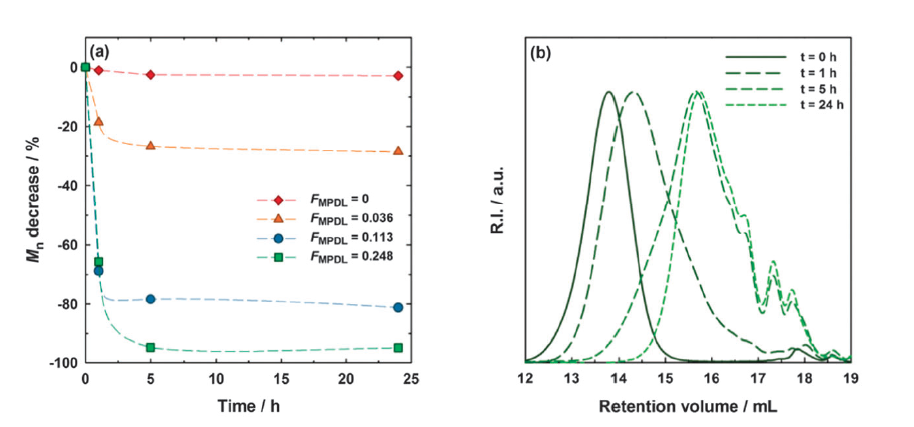
Figure 2. Hydrolytic degradation of P(OEGMA-co-MPDL) at basic pH, as a function of the content in MPDL. (a) Evolution of the number-average molar mass, Mn, over time for different final fractions of MPDL (FMPDL). (b) Example of SEC chromatogram evolution over time (FMPDL=0.248) (from ref 7).
Conclusions: These data demonstrate that the combination of NMP and rROP represents one of the most promising strategies for new tailor-made biodegradable and biocompatible polymers in the biomedical field.
The authors thank the French National Research Agency (ANR-11-JS08-0005) for the financial support of the PhD thesis of VD and the French Ministry of Research for the financial support of the PhD thesis of EG; Arkema is warmly acknowledged for kindly providing the BlocBuilder MA alkoxyamine and the SG1 nitroxide; CNRS is also acknowledged for financial support
References:
[1] J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes, B. Charleux, Nitroxide-mediated polymerization, Prog. Polym. Sci., 38 (2013) 63-235
[2] K. Matyjaszewski, J. Xia, Atom Transfer Radical Polymerization, Chem. Rev., 101 (2001) 2921-2990.
[3] G. Moad, E. Rizzardo, S.H. Thang, Living Radical Polymerization by the RAFT Process – A Second Update, Aust. J. Chem., 62 (2009) 1402-1472.
[4] V. Delplace, J. Nicolas, Degradable Vinyl Polymers for Biomedical Applications, Nature Chem., in press, doi:10.1038/nchem.2343 (2015).
[5] V. Delplace, A. Tardy, S. Harrisson, S. Mura, D. Gigmes, Y. Guillaneuf, J. Nicolas, Degradable and Comb-Like PEG-Based Copolymers by Nitroxide-Mediated Radical Ring-Opening Polymerization, Biomacromolecules, 14 (2013) 3769-3779.
[6] V. Delplace, S. Harrisson, A. Tardy, D. Gigmes, Y. Guillaneuf, J. Nicolas, Nitroxide-Mediated Radical Ring-Opening Copolymerization: Chain-End Investigation and Block Copolymer Synthesis, Macromol. Rapid Commun., 35 (2014) 484-491.
[7] V. Delplace, E. Guegain, S. Harrisson, D. Gigmes, Y. Guillaneuf, J. Nicolas, A ring to rule them all: a cyclic ketene acetal comonomer controls the nitroxide-mediated polymerization of methacrylates and confers tunable degradability, Chem. Commun., 51 (2015) 12847-12850.