Paclitaxel (PTX) has shown its potency against a broad spectrum of cancers. However, its clinical application has been limited owing to poor solubility[1]. Also, PTX cannot be selectively accumulated into tumors tissues. The nonspecific distribution will reduce bioavailability and therapeutic efficiency of PTX[2]. Recently, we have prepared novel silk fibroin modified poly(l-lactide)-poly(ethylene glycol)-poly(l-lactide) (SF/PLLA-PEG-PLLA) composite nanoparticles as drug carrier by solution-enhanced dispersion in supercritical CO2 (SEDS) process[3]. The present study aims to fabricate tumor-targeted SF/PLLA-PEG-PLLA nanoparticle drug delivery systems to decrease adverse side effects of PTX and enhance its therapeutic efficiency.
In this study, PTX loaded SF/PLLA-PEG-PLLA (PTX-SF/PLLA-PEG-PLLA) nanoparticles were prepared by the SEDS process. Then, folic acid (FA), a tumor-specific ligand, was conjugated on the surface of PTX-SF/PLLA-PEG-PLLA nanoparticles with the carboxylic group of FA activated by EDC/NHS. The resulting FA conjugated PTX loaded SF/PLLA-PEG-PLLA (PTX-FA-SF/PLLA-PEG-PLLA) nanoparticles exhibited a spherical morphology with a mean particle size of 665 nm (Figure 1). UV-Vis spectra analysis suggested that the FA content was about 1.67 mg/1 g PTX-FA-SF/PLLA-PEG-PLLA nanoparticles. And the drug load and encapsulation efficiency of PTX-FA-SF/PLLA-PEG-PLLA nanoparticles was 15.5% and 77.4%, respectively. Moreover, the in vitro drug release experiment indicated that PTX-FA-SF/PLLA-PEG-PLLA nanoparticles showed a sustained release and 21.3% of PTX were released in PBS buffer (pH 7.4) in one week (Figure 2). Flow cytometric analysis and the fluorescence microscopy observation indicated that FA-SF/PLLA-PEG-PLLA nanoparticles could be internalized into MCF-7 breast cancer cells and exhibited higher cellular uptake ability than SF/PLLA-PEG-PLLA nanoparticles.These results suggested that PTX-FA-SF/PLLA-PEG-PLLA nanoparticles could be utilized as an effective drug delivery system for tumor targeted therapy.

Figure 1.FE-SEM photograph of PTX-FA-SF/PLLA-PEG-PLLA nanoparticles.
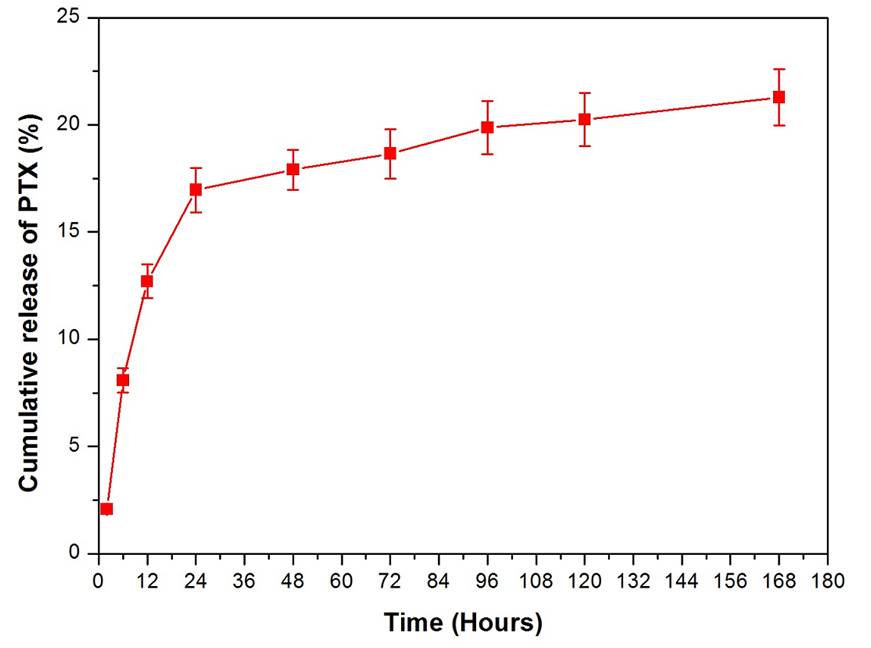
Figure 2.In vitro release profile of PTX from PTX-FA-SF/PLLA-PEG-PLLA nanoparticles in PBS buffer (pH 7.4).
Natural Science Foundation of Hubei Province (No.2014CFB839); Opening Project of Jiangsu Provincial Key Laboratory of Silk Engineering (No.KJS1415); Hong Kong, Macao and Taiwan Science & Technology Cooperation Program of China (No.2015DFH30180)
References:
[1] Danhier F, Danhier P, De Saedeleer CJ, Fruytier AC, Schleich N, des Rieux A, Sonveaux P, Gallez B, Préat V. Paclitaxel-loaded micelles enhance transvascular permeability and retention ofnanomedicines in tumors. Int J Pharm. 2015, 479(2):399-407.
[2] Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002, 235(1-2):179-192.
[3] Zhao Z, Li Y, Zhang Y, Chen AZ, Li G, Zhang J, Xie MB. Development of silk fibroin modified poly(l-lactide)-poly(ethylene glycol)-poly(l-lactide) nanoparticles in supercritical CO2. Powder Technology, 2014, 268, 118-125.