Introduction: Surfaces that have the potential to maintain their clean state by resisting the non-specific binding of proteins are sought after for many applications, from biomedical to surface protection. For many years the gold standard of responsive surfaces has been obtained by grafting short polymer chains of poly(ethylene glycol) (PEG) to the surface due to their well-documented anti-fouling properties. Unfortunately, limited life span and decomposition into toxic compounds reduces the effectiveness of these coatings for industrial paints, creating a need to understand the mechanism behind PEG’s anti-fouling properties and find new and more suitable surface modifiers like poly-2-oxazoline (POX), which has been shown to have a higher life span and more effective anti-fouling behaviour[1] than PEG. Fungal proteins like the class I Hydrophobin EAS[2] present as major foulants for industrial coatings. EAS possesses the ability to self-assemble into stable monolayers, on hydrophobic as well as hydrophilic surfaces[3],[4], due to its distinct amphipathic profile, allowing it to condition the surface to facilitate further microbial growth.
Materials and Methods: We present an all-atom molecular dynamics study on the binding of a class I Hydrophobin, EAS, onto industrial silica surfaces and surfaces modified with PEG and POX. Simulations were performed using the initial set-up shown in Figure 1A for 200 ns using the LAMMPS[5] software with the CHARMM22[6] force-field used for the protein and PEG/POX chains, and the CHARMM-compatible Cruz-Chu[7] silica parameters.
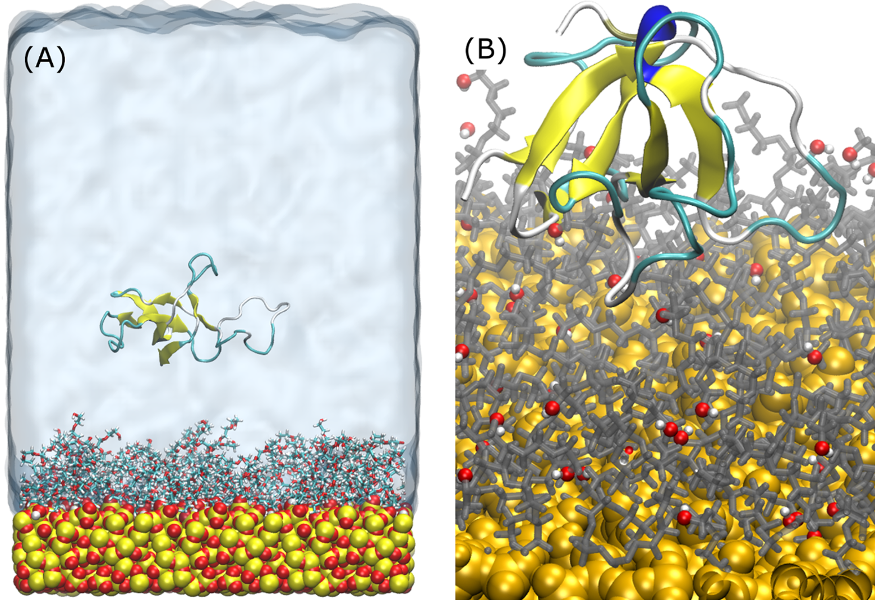
Figure 1 – (A) Example starting configuration of systems. Four different protein starting orientations were examined, with the protein rotated 90o about the y-axis. (B) Typical adsorption of EAS on PEG surfaces. PEG terminals are shown in red (O) and white (H), as can be seen, terminals of PEG near the adsorption site are predominantly collapsed and interacting with the silica surface, encouraging the initial adsorption.
Results and Discussion: In solvent only simulations, POX chains have significantly reduced hydrophobic exposure, as the chains remain in an extended state over time compared to the PEG chains which have a tendency interact with the silica substrate. The hydrophobic exposure of PEG chains, combined with the penetration of hydrophobic residues of EAS is seen to drive the initial protein adsorption on PEG modified surfaces (Figure 1B). For POX systems, we see two prominent adsorption mechanisms. Either the protein extends into cavities in the POX layer, resulting in a strong and almost immobile adsorption, or these cavities fill with water, only allowing the protein to diffuse and rotate on top of the film layer.
Compared to unmodified surfaces, both modified systems show significantly less enhancement of the secondary structure, and the Cys7-Cys8 region responsible for monolayer formation is often kept locked in the protein core, rather than unfolding like it does on the silica surface.
Conclusion: These results provide insight into the nature of anti-fouling polymers and can be used in conjunction with experimental results to help in the rational design of new coatings. Our results highlight the deficiencies presented in PEG modified surfaces, with POX coatings showing significant promise as suitable replacement.
Victorian Life Sciences Computation Initiative; Victorian Partnership for Advanced Computing; National Computational Infrastructure; Pawsey Supercomputing Centre
References:
[1] Ng, CCA, Ciampi, S, Harper, JB & Gooding, JJ, 'Antifouling behaviour of silicon surfaces modified with self-assembled monolayers containing both ethylene glycol and charged moieties', Surface Science, 2010, vol. 604, no. 17-18, 1388-94.
[2] Macindoe, I, Kwan, A., Ren, Q., Morris, V., Yang, W., Mackay, J. and Sunde, M., ‘Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS’ National Academy of Sciences. Proceedings, 2012, vol. 109, no. 14, pp. E804-E811.
[3] Qin, M., Wang, L.K., Feng, X.Z., Yang, Y.L., Wang, R., Wang, C., Yu, L., Shao, B., Qiao, M.Q., Bioactive surface modification of mica and poly(dimethylsiloxane) with hydrophobins for protein immobilization. Langmuir 2007, 23, 4465–4471.
[4] Zhao, Z.X., Qiao, M.Q., Yin, F., Shao, B., Wu, B.Y., Wang, Y.Y., Wang, X.S., Qin, X., Li, S., Yu, L., Chen, Q., Amperometric glucose biosensorbased on self-assembly hydrophobin with high efficiency of enzyme utilization. Biosensors and Bioelectronics 2007, 22, 3021–3027.
[5] Plimpton, S., Fast parallel algorithms for short-range molecular dynamics. Journal of Computational Physics, 1995. 117(1): p. 1-19.
[6] MacKerell, A.D., et al., All-atom empirical potential for molecular modeling and dynamics studies of proteins. The Journal of Physical Chemistry B, 1998. 102(18): p. 3586-3616.
[7] Cruz-Chu, E.R., A. Aksimentiev, and K. Schulten, Water-silica force field for simulating nanodevices. The Journal of Physical Chemistry B, 2006. 110(43): p. 21497-508.