Introduction: Strontium, when incorporated as a network modifier, has been reported to increase the hardness and reduce the dissolution of a glass[1]. Additionally, Sr2+ can stimulate bone formation in vitro[2]. This project was designed to investigate the influence of increasing Sr2+ content on the structure of a borate-based glass series expected to be applied as bioactive coatings on the Ti-6Al-4V implants.
Methods: Glass series were formulated (Table I) by melt quenching. X-ray Diffraction (XRD), Differential Thermal Analysis (DTA) and Raman Spectroscopy were employed to investigate any changes in the glass structure as a result of increasing Sr content.
Results and Discussions: The absence of peaks in the XRD traces confirm the amorphous nature of each glass. The glass transition temperatures (Tg) of the glass series decreased with addition of up to 5 mol% of Sr2+, and then increased with increased additions of Sr2+. It is apparent that the Raman spectra (Figure 1) are dominated by a broad peak centered at 1320 cm-1 and a shoulder at 1500 cm-1. In addition, three Raman bands were detected at 770 cm-1, 860 cm-1 and 940 cm-1. The intensity of the band at 770 cm-1 decreased with increasing Sr2+. However, the intensity of bands at 860 cm-1 and 940 cm-1 increased with Sr2+ content. The symmetric breathing vibration of [BØ4] is assigned at around 770 cm-1[3]. The increasing intensity of Raman bands at 860 cm-1 and 940 cm-1 and the larger peak areas under 860 cm-1, 1320 cm-1 and 1500 cm-1 indicate increased presence of [BØ2O]- and [B2ØO4]4- group introduced by non-bridging oxygen (NBO) within the glasses as more Sr2+ is incorporated. [3] Tg of borate glasses increased with the increase Metal-oxygen boding (FR-O). Therefore, more ionic Sr-O bonding increases Tg of the glass series.
Table 1. Compositions of the glass series (mol%).
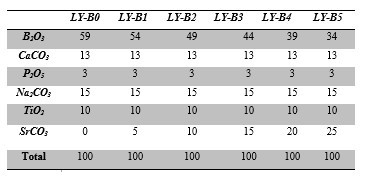
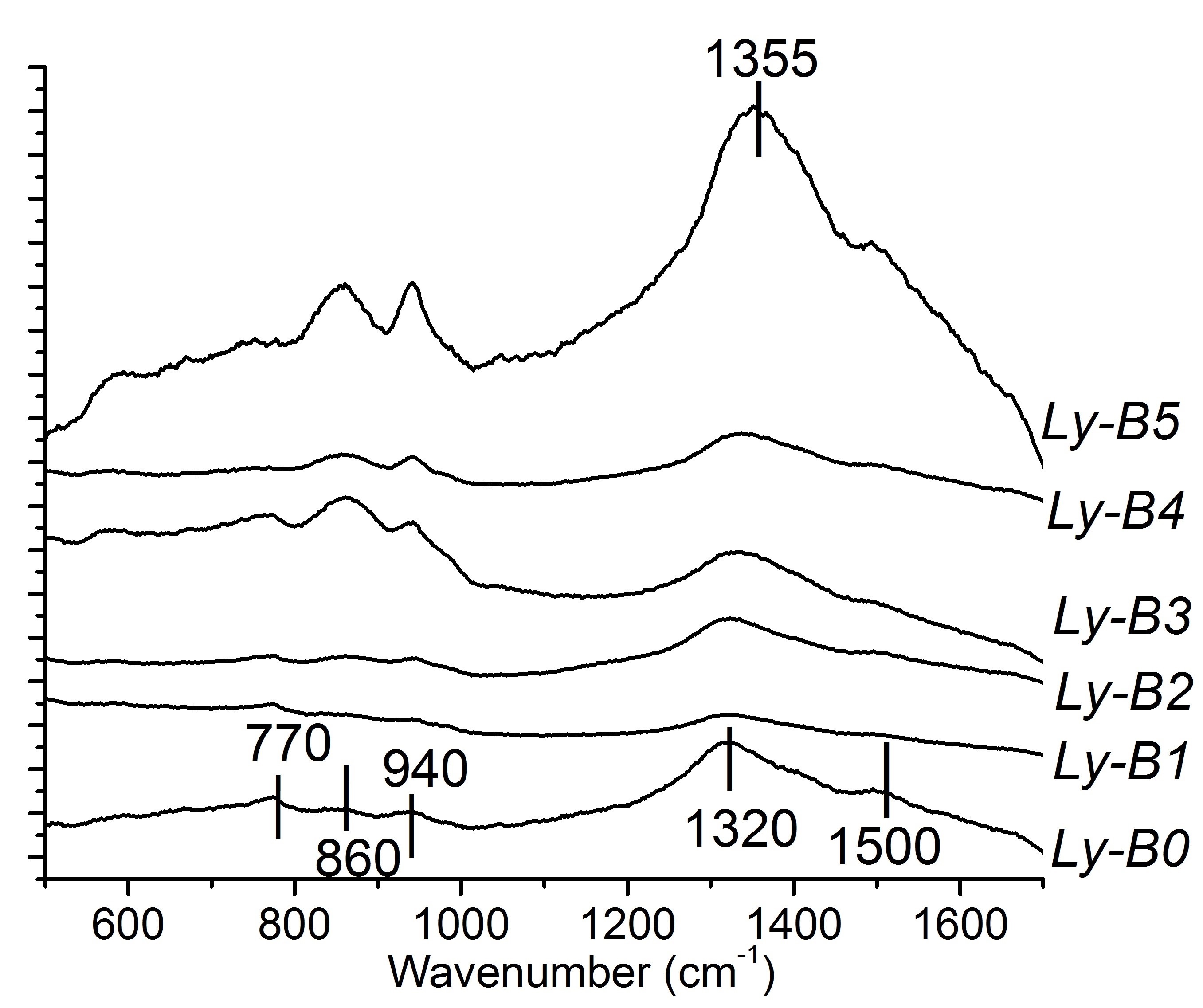
Figure 1. Raman spectroscopy of the borate glass series.
Conclusions: Increasing Sr2+ content induced higher amounts of NBO expected to decrease Tg. However, Tg increased with the addition of more than 5 mol% of Sr2+. This can be explained by the fact that enough Sr2+was present to induce more Sr-O bonding providing additional cross-links across borate segments of the glass network.
The Canadian Institute of Health Research (CIHR); The Natural Sciences and Engineering Research Council of Canada (NSERC) through the Collaborative Health Research Project (CHRP) program (grant #315694-DAN)
References:
[1] Li, Y., Coughlan, A., Laffir, F.R., Pradhan, D., Mellott, N.P., and Wren, A.W., Investigating the mechanical durability of bioactive glasses as a function of structure, solubility and incubation time. Journal of Non-Crystalline Solids, 2013. 380: p. 25-34
[2] O’Donnell, M., Candarlioglu, P.L., Miller, C.A., Gentleman, E., and Stevens, M.M., Materials characterisation and cytotoxic assessment of strontium-substituted bioactive glasses for bone regeneration. Journal of Materials Chemistry, 2010. 20(40): p. 8934-8941.
[3] Yiannopoulos, Y., Chryssikos, G.D., and Kamitsos, E., Structure and properties of alkaline earth borate glasses. Physics and Chemistry of Glasses-European Journal of Glass Science and Technology Part B, 2001. 42(3): p. 164-172