Borophosphosilicate glass – Polycaprolactone hybrid scaffolds for bone tissue engineering
-
1
The University of Western Ontario, Chemical and Biochemical Engineering, Canada
-
2
The University of Western Ontario, Bone and Joint Institute, Canada
-
3
The University of Western Ontario, Dentistry, Schulich School of Medicine & Dentistry, Canada
Introduction: Scaffold materials for bone tissue engineering applications should be osteoconductive and osteoinductive while exhibiting suitable porosity to allow cell infiltration, tissue growth and metabolic waste removal. Their rate of degradation must match the rate of tissue formation [1]-[3]. Conventional bioactive composite materials have been proposed to be a solution to this problem [4]-[5]. However, these materials possess micro-scale domain sizes leading to distinct phases. This, in turn, results in their non-uniform physical, chemical, mechanical and biological properties at the nano or molecular level making them unsuitable for bone biomaterials [6]. The main objective was to synthesize and characterize a range of bioactive organic-inorganic class II hybrid biomaterials via a non-aqueous sol-gel process with poly (ԑ-caprolactone) diol (PCL) as the organic component and SiO2-P2O5-B2O3 based ternary Borophosphosilicate glass (BPSG) as the inorganic component.
Materials and methods: PCL diol was end-capped with 3-Glycidoxypropyl trimethoxysilane then reacted with metal alkoxides in a non-aqueous sol-gel process to produce PCL-BPSG hybrid biomaterials. Fibrous scaffolds of the synthesized hybrids biomaterials were electrospun from the viscous sol.
Results and discussion: To study organic-inorganic bridging, solid-state 29Si-MAS NMR was conducted for BPSG and 50 wt% PCL-50 wt% BPSG (50H) hybrid biomaterials (Figure 1A).
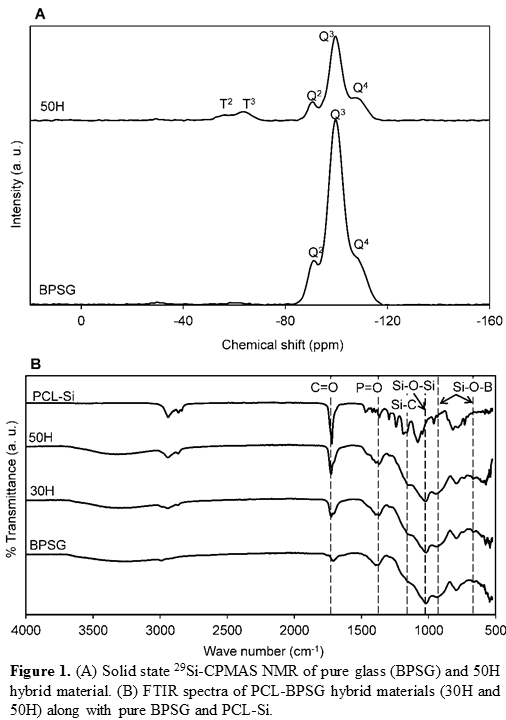
In silica networks, if the silicon atom is bonded to four oxygen atoms, then the resultant structure is designated as Q network. However, if a silicon atom is bonded to three oxygen atoms and one carbon atom, then the structure is designated as T network. Both of BPSG and 50H exhibited quaternary Si-O-Si bridging networks, which is denoted as Q4 (superscript indicates the number of Si-O-Si framework connections with respect to the silicon atom). Chemical shifts at δ= -92, -100 and -109 ppm were associated with Q2, Q3 and Q4 structures respectively. Chemical shifts at δ= -57 and -64 ppm denoted as T2 and T3 were attributed to the Si-O-Si bridging networks with Si-C at one end. The control BPSG did not have any peak for T networks because it has no Si-C bonding associated with Si-O-Si bridging network. In contrast, 50H spectrum showed a T3 network since Si-C bond from PCL-Si is covalently bonded with the inorganic glass network and resulted in a class II hybrid. In the FTIR spectra of PCL-BPSG hybrids (Figure 1B), characteristic peak at 1072 cm-1 is attributed to Si-O-Si stretching and the peaks at 920 and 670 cm-1 are attributed to Si-O-B stretching vibration. 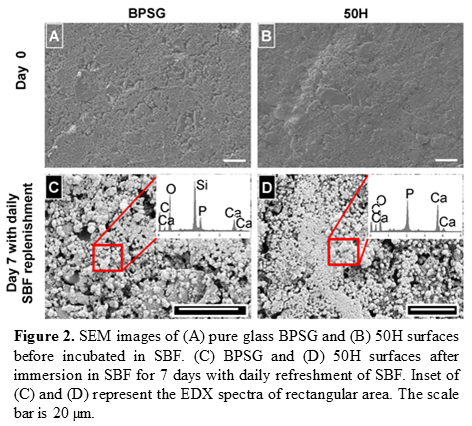
SEM images of BPSG and 50H surface before and after incubation in simulated body fluid (SBF) for 7 days are shown in Figure 2. Before incubation in SBF, BPSG and PCL-BPSG hybrid exhibited smooth surfaces. After incubation for 7 days both BPSG and PCL-BPSG surfaces were covered by spherical hydroxyapatite particles. From EDX spectrum (inset of Figures 2C and 2D) the Ca/P ratio were 1.7±0.6. The fibrous scaffolds from hybrid biomaterials produced by electrospinning had diameter ranged between 100 -150 nm (figure not shown).
Conclusion: PCL-BPSG class II hybrid biomaterials were successfully synthesized via a non-aqueous sol-gel process. Fibrous scaffolds of the synthesized hybrids were successfully produced by electrospinning. Hydroxyapatite deposition was observed on the hybrid materials following incubation in SBF. The synthesized hybrid biomaterials could be potential candidates for bone tissue engineering.
Vanier Canada Graduate Scholarship; Natural Sciences and Engineering Research Council of Canada
References:
[1] Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546-554
[2] Hutmacher, D. W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529-2543
[3] O'Brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88-95.
[4] Niemelä, T.; Niiranen, H.; Kellomäki, M.; Törmälä, P. Self-reinforced Composites of Bioabsorbable Polymer and Bioactive Glass with Different Bioactive Glass Contents. Part I: Initial Mechanical Properties and Bioactivity. Acta Biomater. 2005, 1, 235-242.
[5] Rezwan, K.; Chen, Q. Z.; Blaker, J. J.; Boccaccini, A. R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413-3431.
[6] Jones, J. R. Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457-4486.
Keywords:
biomaterial,
nanofiber,
Bioactivity,
3D scaffold
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials in musculoskeletal orthopeadics and tissues
Citation:
Mondal
D,
Mequanint
K and
Rizkalla
AS
(2016). Borophosphosilicate glass – Polycaprolactone hybrid scaffolds for bone tissue engineering.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00815
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.