Introduction: In the last two decades significant efforts have been made to develop soft materials exploiting the self-assembly of short peptides for biomedical applications[1]. β-sheet forming peptides have been shown to allow the design of highly stable shear thinning hydrogels with potential application is a range of fields[2],[3]. In particular, they offer a flexible platform for the design of injectable 3D scaffolds for drug and cell delivery[4],[5].

Figure 1: Schematic representation of the self-assembly process of β-sheet forming peptides
Materials and Methods: We have developed a platform for the design of hydrogels with tailored properties and functionalities exploiting the self-assembly of short (4-10 amino acids) β-sheet forming peptides. The design of these peptides is based on the alternation of hydrophilic and hydrophobic residues. These novel materials have been characterized using a variety of techniques including small angle scattering and rheology.
Results and Discussion: The self-assembly process of these peptides was investigated and is schematically described in Figure 1. By altering the peptide primary structure, the formulation and the processing conditions the properties of these scaffolds (e.g.: shear thnning and recovery, modulus and functionality) can be easily controlled. We were able to design injectable, as well as sprayable, hydrogels that can be used for 3D cell culture as well as in-vivo cell delivery.
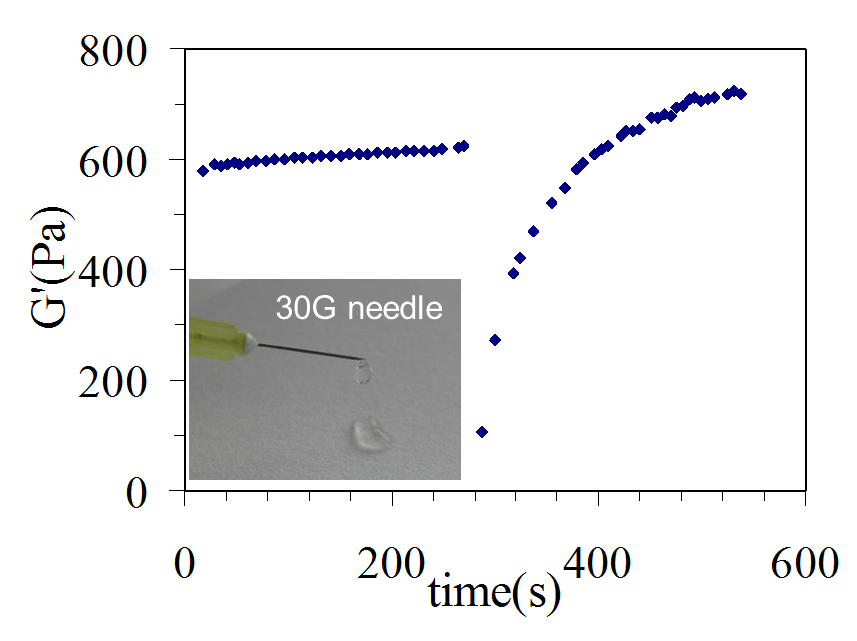
Figure 2: G' vs time showing the shear thinning properties of the hydrogels and therefore their injectbility. Injection was simulated by continuously shearing the hydrogel in-situ in the rheometer for 30 seconds.
We have used these novel materials for the culture of a variety of cells including chondrocytes[4], osteoblasts[5], as well as embryonic stem cells. These hydrogels were then used in a rat infact in-vivo model and shown to induce low immune response and allow the delivery of cardiac progenitor cells. Evedence for their differentiation into cardiomyocites in-situ was obtained.
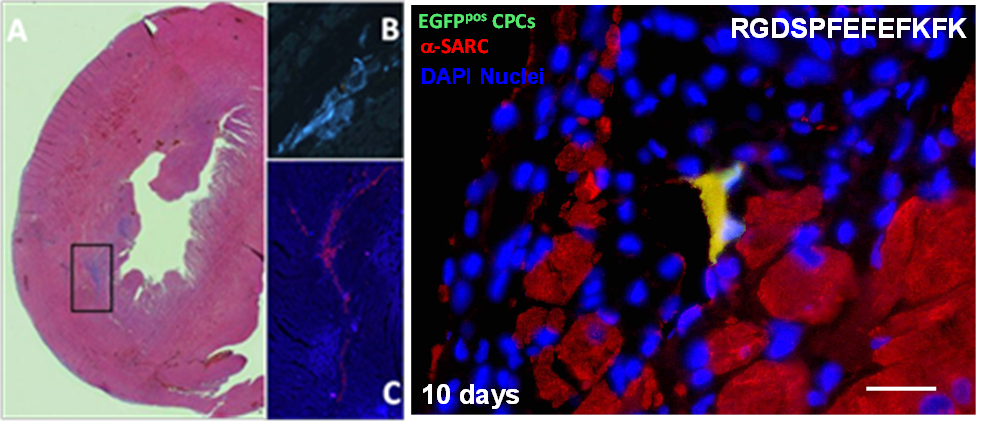
Figure 3: Gel injection in rat heart (left) and evidence of rat cardiac progenitor cell delivered differenciating in the miocardium (right).
Conclusions: We have developed a platform for the design of 3D scaffolds whose properties can be tailored to accommodate different cells’ needs. Our results clearly demonstrate that our peptides offer great promise for the design of specific cell niches due to their low immunogenicity and the ability we have to control and tailor their properties.
UK Engineering and Physical Sciences Research Council (EPSRC) Research Fellowship Grant no: EP/K016210/1; European Union FP7 Framework Programme Bioscent Grant no: 214539
References:
[1] J. Collier et al. Chem. Soc. Rev., 39, 3413 (2010)
[2] A. Saiani et al. Soft Mat., 5, 193 (2009)
[3] D. Roberts et al. Langmuir, 28, 16196 (2012)
[4] A. Mujeeb et al. Acta Biomat, 9, 4609 (2013)
[5] C Diaz et al. Journal of Tissue Engineering, 5, 2041731414539344 (2014)