Introduction: A kidney transplant is the current gold standard of treatment for end-stage renal disease and the need for renal replacement, but the severe shortage of transplantable donor kidneys limits the availability of this procedure[1]. Kidney bioengineering and renal regeneration have the potential to solve this issue[2],[3], but a greater understanding of the complex role of the extracellular matrix (ECM) in directing kidney-specific cellular response and interactions is required to promote advances in such projects. In this study we have developed a hydrogel composed of reconstituted renal ECM and demonstrate the ability of this material to promote cell proliferation with evidence that the material may be able to direct cellular response.
Materials and Methods: Porcine kidneys were chopped up into chunks and decellularized via alternating washes of sodium dodecyl sulfate and water. Decellularization efficiency was determined by histology of native and decellularized tissue, and remaining DNA content as measured by the PicoGreen Assay. The isolated ECM was lyophilized, milled, and subjected to a pepsin digestion. To form a hydrogel, the pepsin digest was neutralized, buffered, brought up to volume, and allowed to set at 37°C for at least 1 hr to ensure complete gelation[4]. Renal cortical tubular epithelial (RCTE) cells were seeded and cultured on top of gels for up to 16 days. Live/Dead staining and confocal imaging of cells were used to assess cell proliferation and viability. Cells cultured on tissue culture plastic served as a control group.
Results: Histology of native and decellularized tissue (Figure 1A,B) revealed complete removal of nuclei and few remaining cellular debris in decellularized tissue sections.
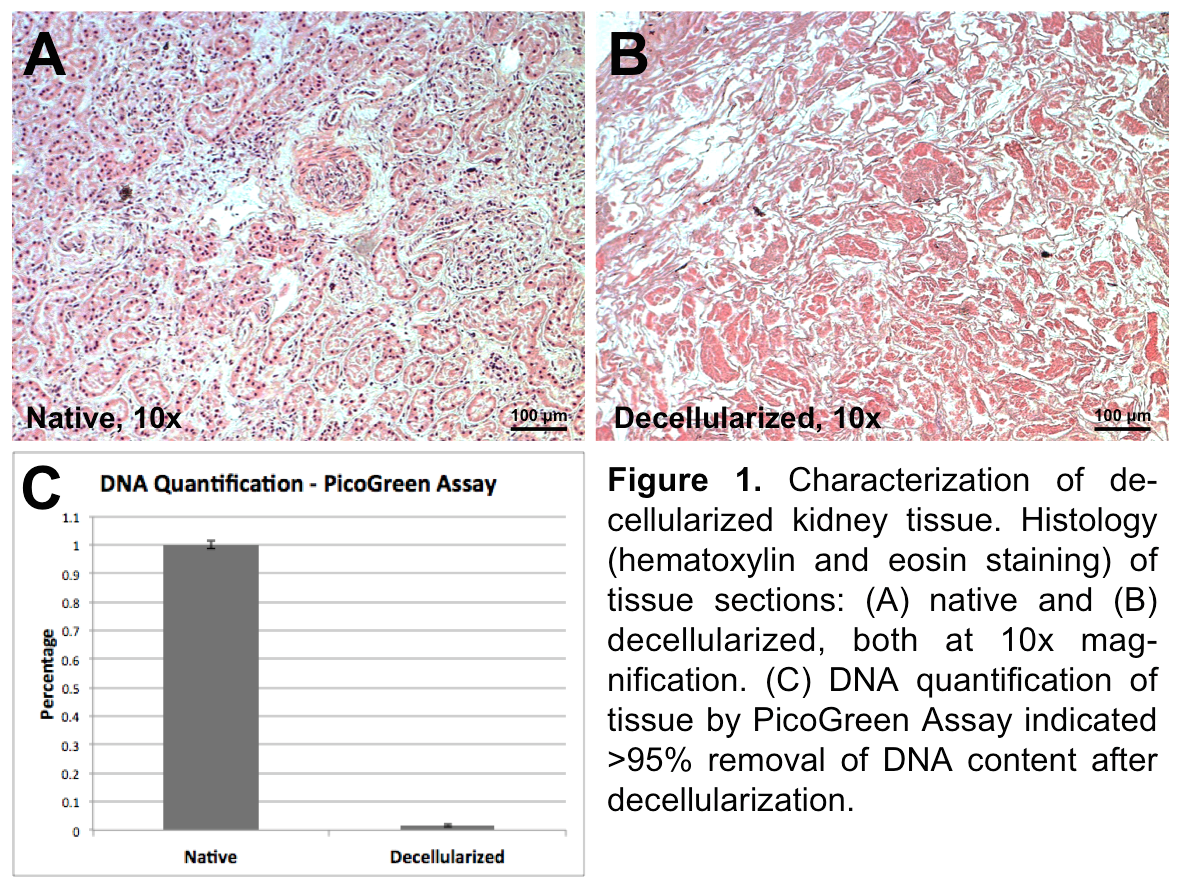
PicoGreen Assay of samples (Figure 1C) indicated >95% removal of DNA content in decellularized tissues.
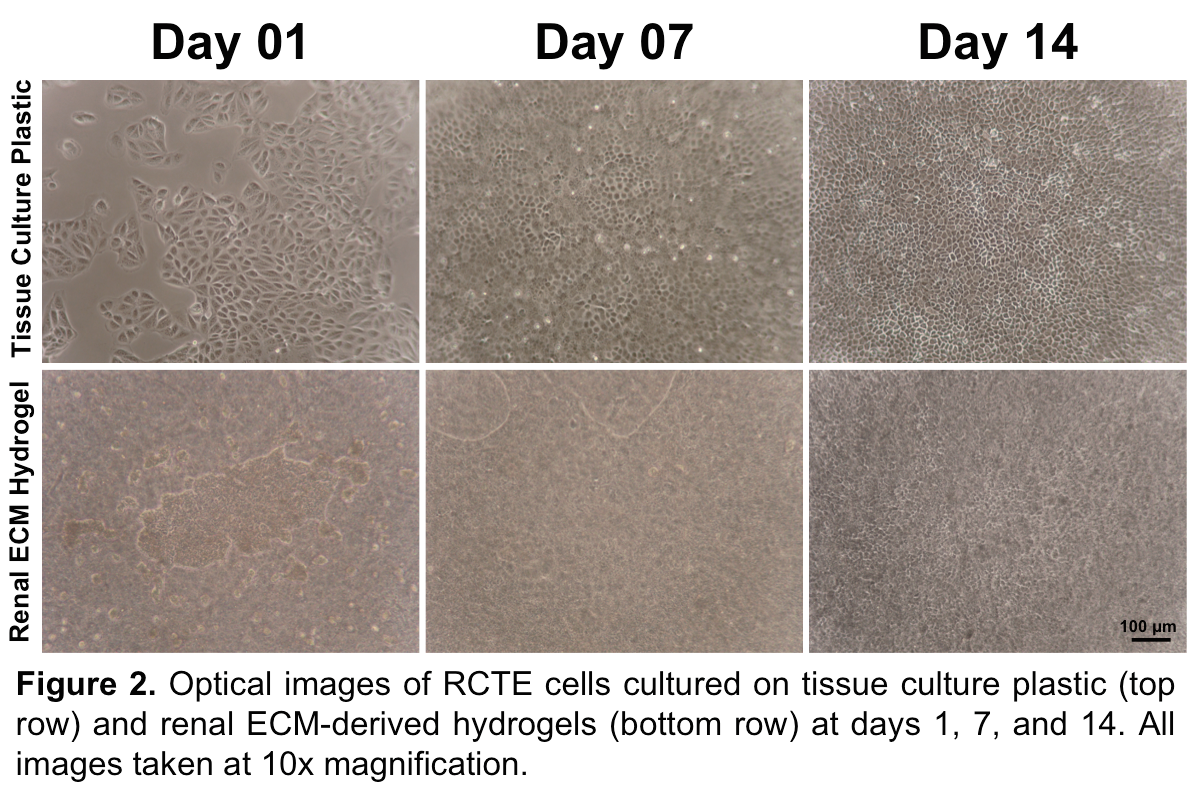
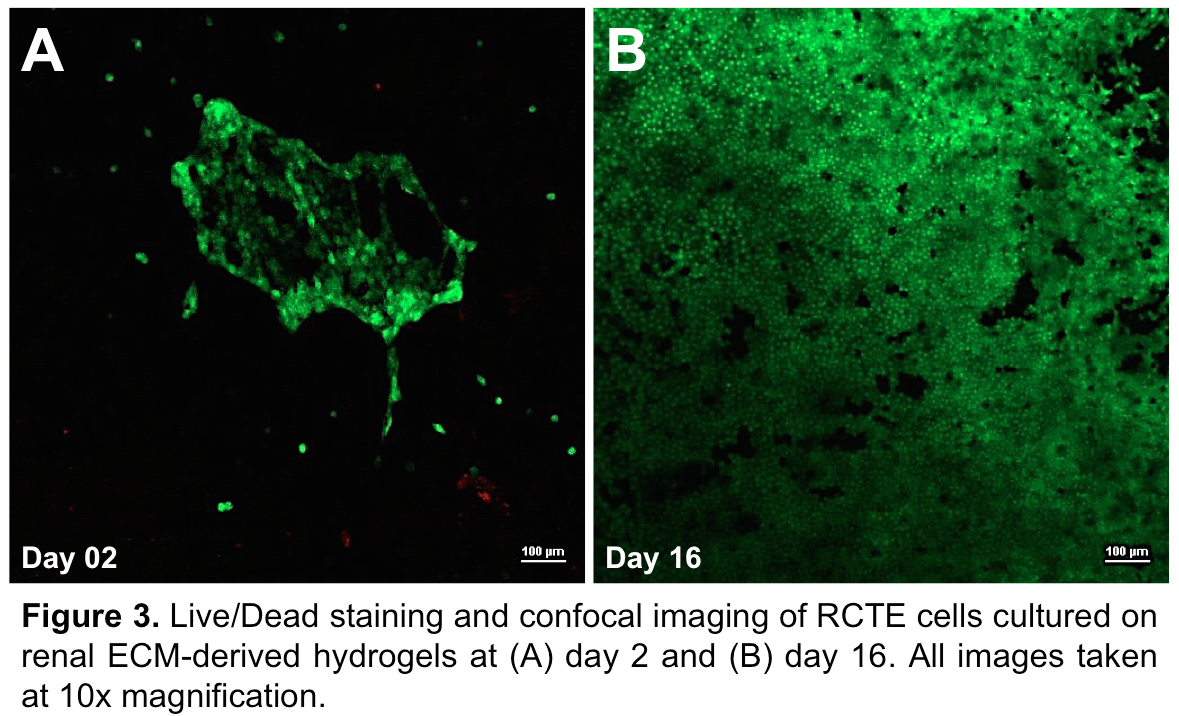
High cell density and good cell viability were found on gels throughout the culture period as can be seen by optical images (Figure 2) and Live/Dead staining of cells cultured on 0.5 wt% hydrogels (Figure 3).
Discussion and Conclusion: Here we demonstrate that isolated renal ECM can be processed into hydrogels capable of supporting cell proliferation and growth. Interestingly, cells cultured on these gels exhibit a morphology distinct from those cultured on tissue culture plastic, suggesting the mechanical properties and protein composition of the gels impact cellular response. The material we have developed has the potential to advance understanding of cell-matrix interactions by providing a method for cell culture and possibly encapsulation within a microenvironment composed of native renal ECM proteins. Further investigation is required to determine the influence of composition and concentration of hydrogels on cell-specific response. Future work includes developing a 3D-printable hydrogel material for scaffold fabrication and cell printing, providing a cell-instructive environment for renal regeneration and kidney bioengineering.
We would like to thank Dr. Heather H. Ward and Dr. Angela Wandinger-Ness from the University of New Mexico for kindly providing the RCTE cells.; We also gratefully acknowledge Alexandra L. Rutz and Phillip L. Lewis from Northwestern University for technical assistance with hydrogel formulation and setup.; Imaging work was performed at the Northwestern University Center for Advanced Microscopy generously supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.; We also would like to acknowledge use of the Simpson Querrey Institute for BioNanotechnology Analytical BioNanoTechnology Equipment Core developed by support from the U.S. Army Research Office, the U.S. Army Medical Research and Material Command, and Northwestern University.; This work was additionally supported by the Northwestern University Mouse Histology and Phenotyping Laboratory and a Cancer Center Support Grant (NCI CA060553).; We thank the support of the Zell Family Foundation.; We recognize the Career Development Award from the Robert R. McCormick Foundation, the Northwestern Memorial Foundation Dixon Translational Research Grants Initiative, the American Society of Transplant Surgeon's Faculty Development Grant, and a Research Grant for the Young Investigator from the National Kidney Foundation of Illinois.; This work was also supported by NIDDK K08 DK10175 to J.A.W., NIDDK 1K01 DK099454-01 to R.N.S., and the Northwestern University Biotechnology Training Program (NIGMS T32 GM008449) for J.S.
References:
[1] 2014 Annual Data Report. US Renal Data System: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
[2] Uzarski JS, Xia Y, Belmonte JCI, and Wertheim JA. “New strategies in kidney regeneration and tissue engineering.” Current Opinion in Nephrology and Hypertension 23 (2014): 399-405.
[3] Kim S, Fissell WH, Humes HD, and Roy S. “Current strategies and challenges in engineering a bioartificial kidney.” Frontiers in Bioscience (Elite Edition) 7 (2015): 215-228.
[4] Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers C, D’Amore A, Nagarkar SP, Velankar SS, and Badylak SF. “A hydrogel derived from decellularized dermal extracellular matrix.” Biomaterials 33 (2012): 7028-7038.