Tissue-engineered skeletal muscle with collagen sponge scaffold having contractile properties
-
1
Osaka Institute of Technology, Department of Biomedical Engineering, Japan
Introduction: A tissue-engineered skeletal muscle is a promising strategy for the reconstruction of skeletal muscle loss caused by tumour ablations and by accidental injury. In the process of skeletal muscle regeneration, myoblasts fuse each other to form multinucleated myotubes, and then the myotubes mature to myofiber in vivo. The primary function of skeletal muscle is to generate force. We have already prepared the tissue-engineered skeletal muscle of three-dimensional structure having diameter of about 0.5 mm and length of 12 mm by the collagen gel cell culture. However, it is still a big challenge to construct a matured muscle tissue in vitro having applicable mass to tissue transplantation. As tissue constructs increase in scale, their size is limited by poor nutrient supply. The current major issue is a lack of angio architecture in the tissue-engineered constructs. In this study, a collagen scaffold having uniaxial porous structure was reported for the large-scaled and has function native-like contractile properties by electrical stimulations for tissue-engineered skeletal muscle.
Materials and Methods : The polytetrafluorethylene tube was filled with Type I collagen gel solution. It was placed vertically and frozen by gradual immersion into liquid nitrogen from its bottom to top. The frozen collagen gel solution was then dried in a vacuum oven. The obtained sponge scaffold was subjected to the SEM observation. The C2C12 established myoblasts were seeded into the collagen sponge scaffold. The construct was cultured for 2 days in DMEM containing fetal bovine serum and then the medium was changed to DMEM containing horse serum to enhance differentiation of the cells to the myotubes. The contractile force of tissue-engineered skeletal muscle caused by electric stimulation was measured after cultures. Then the construct was observed histologically by HE staining.
Results and Discussion: The obtained collagen sponge was shown to have unidirectional porous structure. This structure may be created by ice crystals formed vertically in the collagen solution by gradual freezing by liquid nitrogen. The C2C12 cells were dispersed throughout the structure of the collagen sponge. However tissue-engineered skeletal muscle observed C2C12 cells gradually becomes one line. Tissue-engineered skeletal muscle the reaction was in response to the twitch and tetanus stimulation applied.
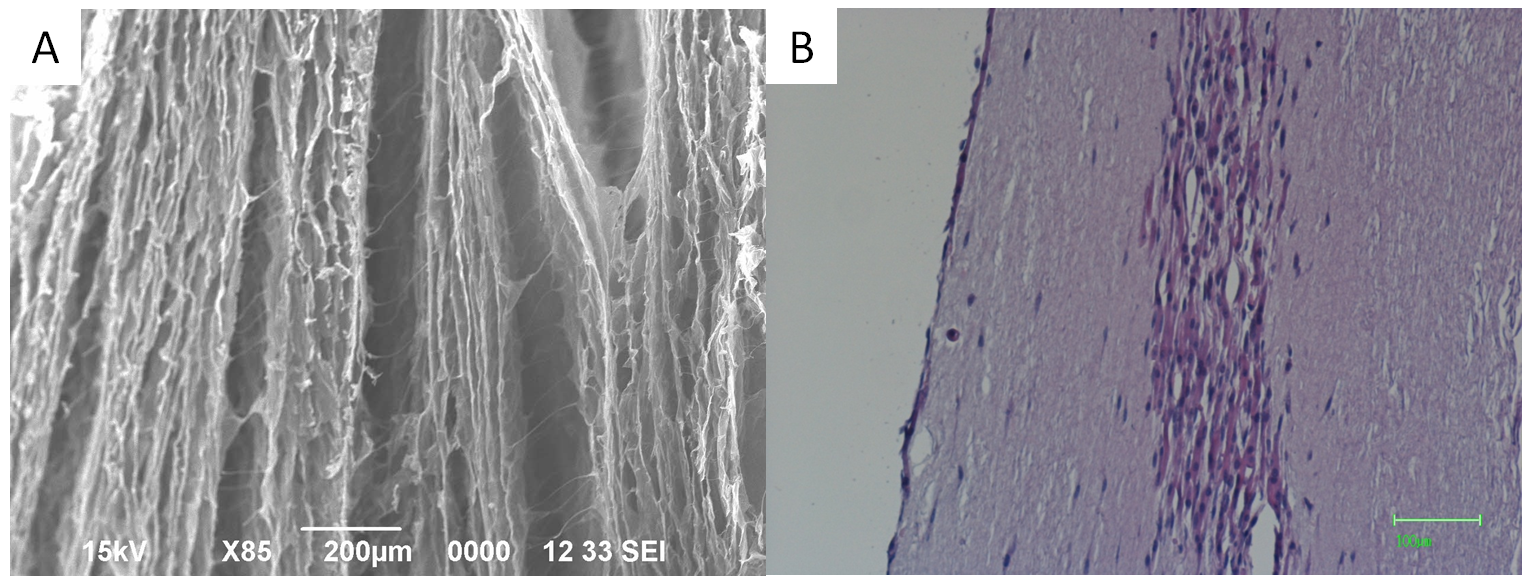
Fig.1 Histological evaluation of collagen sponge scaffold. (A) The SEM image of collagen sponge scaffold. Bar = 200 µm. (B) The HE staining of cultured tissue-engineered skeletal muscle. Bar = 100 µm.
Conclusions: From these results, the collagen sponge having unidirectional porous structure may be useful for giving large mass tissue engineered constructs. Collagen sponge has native-like contractile properties by electrical stimulations.
Keywords:
Cell Differentiation,
Tissue Engineering,
in vivo
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials in constructing tissue substitutes
Citation:
Takagi
S,
Nakamura
T and
Fujisato
T
(2016). Tissue-engineered skeletal muscle with collagen sponge scaffold having contractile properties.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00651
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.