Introduction: The amoeba Naegleria fowleri is the causative agent of Primary Amebic Meningoencephalitis (PAM), which results in a fatal hemorrhagic necrotizing meningoencephalitis[1]. In spite of advances in treatment, PAM is almost always fatal[2]. In an attempt to improve therapeutic delivery to this pathogen, we have used polyanhydride nanoparticles to increase penetration of drugs into the amoeba. Polyanhydride nanoparticles based on sebacic acid (SA), 1,6-bis(p-carboxyphenoxy)hexane (CPH), and 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) have been shown to be effective drug carriers in previous work[3]. Inhibition of amoeba growth and metabolic activity was significantly greater when treated with nanoparticles loaded with rifampicin compared to that of amoeba treated with drug alone.
Methods: N. fowleri (ATCC 30174) was maintained in Nelson’s media at 37ºC and 5% CO2. Imaging of rhodamine-loaded 20:80 CPTEG:CPH nanoparticles within amoeba was performed on 4% PFA fixed cells using confocal laser scanning microscopy. Internalization was assessed at two hours with 24-hour intervals thereafter. Prior to fixation the amoeba were stained with Cell Tracker Green. The coverslips were mounted onto slides with Prolong Gold Antifade Mountant with DAPI.
The in vitro efficacy of drug loaded nanoparticles was compared to that of the soluble drug by employing direct microscopic counts using Trypan Blue exclusion and indirectly through metabolic activity by incubating with Alamar Blue.
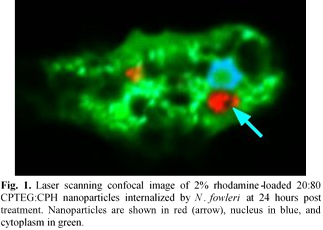
Results and Discussion: Internalization of nanoparticles by the amoeba was pronounced at two hours post treatment, and by 24 hours approximately 90% of amoeba had internalized nanoparticles. The internalized nanoparticles appear to reside within vacuoles within the amoeba (Fig. 1). In vitro treatment with 25 μg/mL rifampicin-loaded 20:80 CPTEG:CPH nanoparticles significantly inhibited the growth of the amoeba by 24 hours post treatment. At all post treatment time points studied, the rifampicin-loaded nanoparticles significantly inhibited growth of amoeba compared to soluble rifampicin (Fig. 2). In contrast to the experiments with rifampicin, in vitro delivery of amphotericin B did not demonstrate the same benefits of nanoparticle encapsulation. These differences in inhibition between rifampicin and amphotericin B could be explained by the different mechanisms of action of these two drugs. Amphotericin B acts upon ergosterol within the cell membrane, whereas rifampicin inhibits RNA synthesis. This suggests that intracellular delivery of rifampicin (enabled by the nanoparticles) would improve its efficacy, while in the case of amphotericin B, intracellular delivery would be unnecessary because the drug acts directly upon the cell membrane.
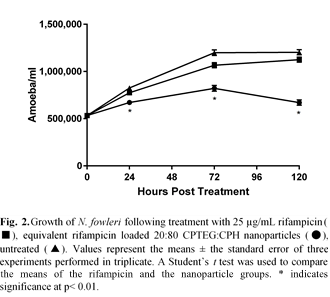
Conclusions: These results indicate that encapsulating rifampicin within polyanhydride nanoparticles improved in vitro amoebastatic efficacy against N. fowleri and may improve patient survival by incorporating into PAM treatments.
References:
[1] F. Marciano-Cabral, G. A. Cabral, FEMS. Immunol. Med. Microbiol. 51, 243-259 (2007).
[2] J. S. Yoder, B. A. Eddy, G. S. Visvesvara, L. Capewell, M. J. Beach, Epidemiol. Infect. 138, 968-975 (2010).
[3] B. R. Carrillo-Conde, R. J. Darling, S. J. Seiler, A. E. Ramer-Tait, M. J. Wannemuehler, and B. Narasimhan, Chem Eng Sci, 125, 98-107 (2015).