Introduction: Polymeric nanoparticles (NPs) are a promising therapeutic strategy for cancer due to their capacity to overcome biological barriers. In addition, encapsulation of chemotherapeutic drugs, such as paclitaxel (PTX), inside targeted nanoparticles can further increase the therapeutic index by delivering an elevated dose directly to a tumor while decreasing systemic toxicity[1]. In the case of brain tumors, a careful engineering of NPs is needed to enable the blood brain barrier (BBB) crossing and to promote receptor mediated uptake into cancer cells[2]. The aim of our project is to develop a targeted PTX delivery nanomaterial for the treatment of glioblastoma.
Materials and Methods: To achieve our objective, proprietary polymers were synthesized and used to manufacture nanoparticles using the nanoprecipitation method[2]. NPs were characterized in terms of: size, surface charge (by DLS), drug content and peptide decoration (by UPLC). Following, in vitro studies, including cytotoxicity assays, haemolysis tests and pyrogenic content analysis, were performed to assess their safety and efficacy. Finally, the BBB penetrating capacity of the NPs was determined by in situ brain perfusion studies and in vivo efficacy studies were performed on a murine orthotopic model for human glioblastoma.
Results and Discussion: Nanoparticles were prepared by the nanoprecipitation method. It was possible to scale up the manufacturing process (from 20mg to 800mg) without modifying NPs properties: mean size was around 100-120 nm (PDI < 0.2) and surface charge was slightly negative, around -10 mV, being both properties appropriated for the intravenous (iv) administration and further BBB crossing[3]. The amount of the BBB penetrating peptide functionalizing nanoparticles was around 0.2% and their drug content 2wt%. In vitro studies showed that peptide-decorated PTX-loaded NPs produced a high cytotoxicity, similar to free PTX onto glioma cells, while they did not affect astrocytes and endothelial cells survival. Furthermore, NPs showed no hemolytic behavior and their pyrogen content was below the accepted limit for iv administration. Moreover, in situ brain perfusion studies confirmed the BBB penetration capacity of our nanoparticles in comparison to free PTX. Finally, in vivo efficacy studies demonstrated that peptide-decorated PTX-loaded NPs resulted in a significant delay of the tumor growth compared to the control group, treated with empty NPs and, in addition, they significantly increased animal life span by 25% (Figure 1).
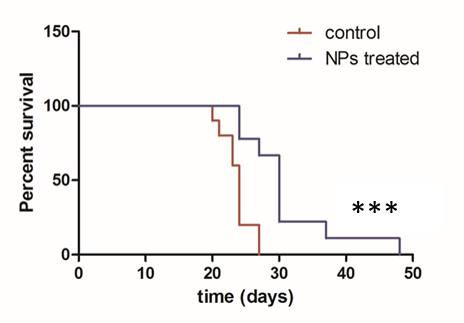
Conclusion: Our peptide-decorated PTX-loaded NPs are able to cross the BBB and target glioblastoma cells. Therefore, they represent a promising alternative for the treatment of brain tumors.
References:
[1] R. Langer, Nature 392, 5–10 (1998)
[2] K. Bouchemal, C. Vauthier, Pharm Res 26(5), 1025 – 1058 (2009)
[3] M.A. Dobrovolskaia, S. McNeil, Nature Nanotechnol 4, 411 - 414 (2009)