Introduction: Bacterial cellulose (BC), produced conventionally by the aerobic Acetobacter genera of bacteria, most notably the Gluconacetobacter xylinus bacterium[1], is a natural nanobiomaterial that has found many biomedical uses such as scaffolds in the repair and regeneration of skin, blood vessel, cornea, heart valve prosthesis, urethra, bone, cartilage and knee menisci, as well as for the delivery of drugs, hormones and proteins[2]. Since G. xylinus is a strict aerobe, oxygen mass transfer is often a limiting factor and the fermentation process usually takes up to 3 weeks in static culture[3]. The relatively low BC productivity by G. xylinus greatly inhibits the realization of its commercial applications[4]. Current strategies for increasing production are complicated and costly although its production in a stirred tank bioreactor has been demonstrated[5]. Recently, a novel facultative bacterium that is capable of producing BC at a significantly higher rate has been discovered. Its growth and BC production may not be limited by dissolved oxygen availability. This paves the way for large scale BC production, an important step towards the realization of many of the biomedical applications envisioned.
Materials and Methods: BC was produced in static culture by the novel bacterium at room temperature (21°C) for 12 to 24 h. To remove bacterial cells after fermentation, 1% NaOH was used to treat BC pellicle at 80°C for 3 h. The bacteria were observed with confocal and fluorescence microscopes. The morphological, chemical/structural and mechanical properties of the BC produced were characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), X-ray diffraction (XRD) and energy dispersive X-ray spectroscopy (EDX) and atomic force microscopy (AFM). The properties of the BC were compared to that produced by G. xylinus. A method adapted from our recently issued patent was used to coat silver nanoparticles on BC[6]. The antimicrobial properties of the BC were assessed.
Results and Discussion:
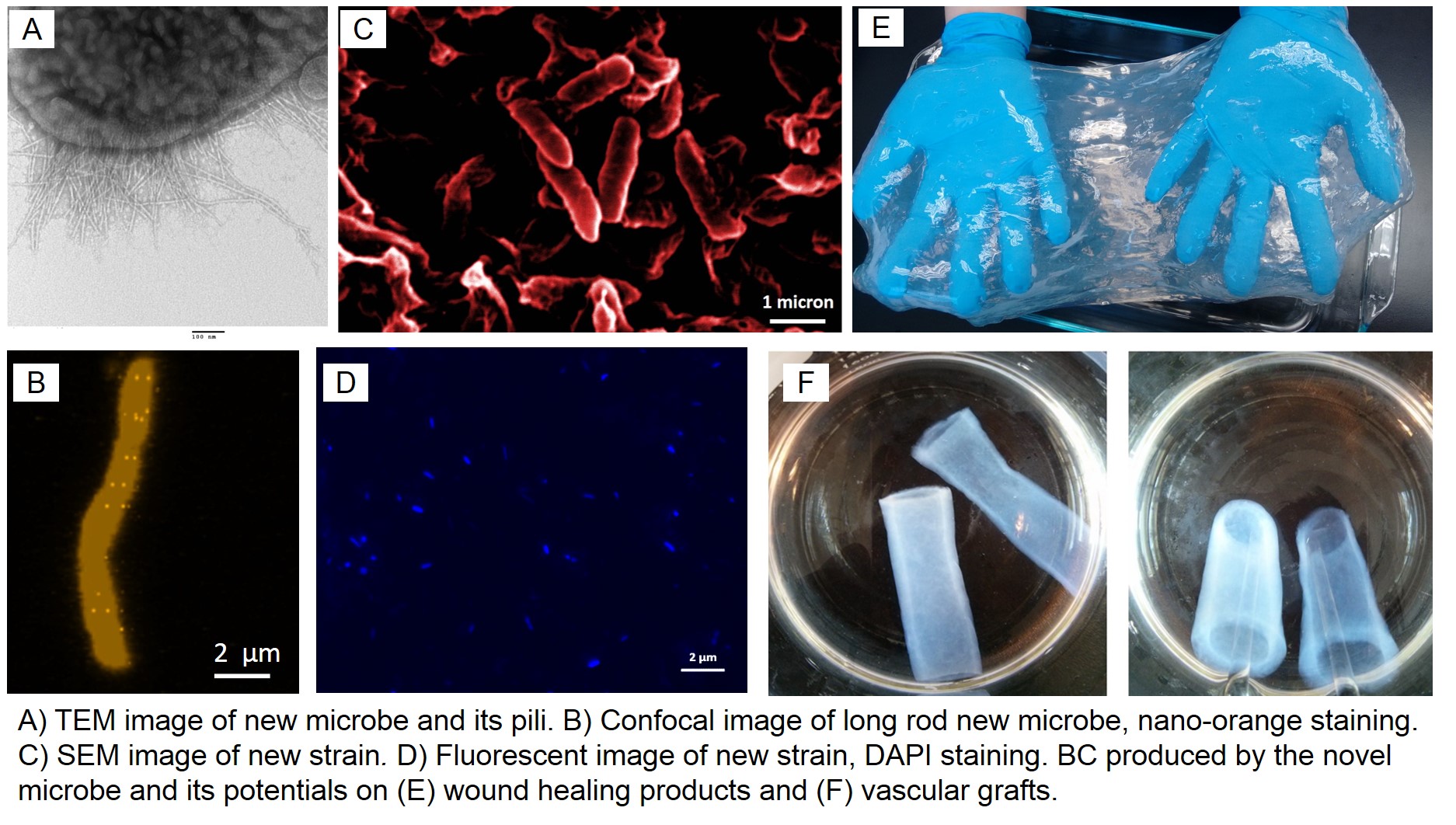
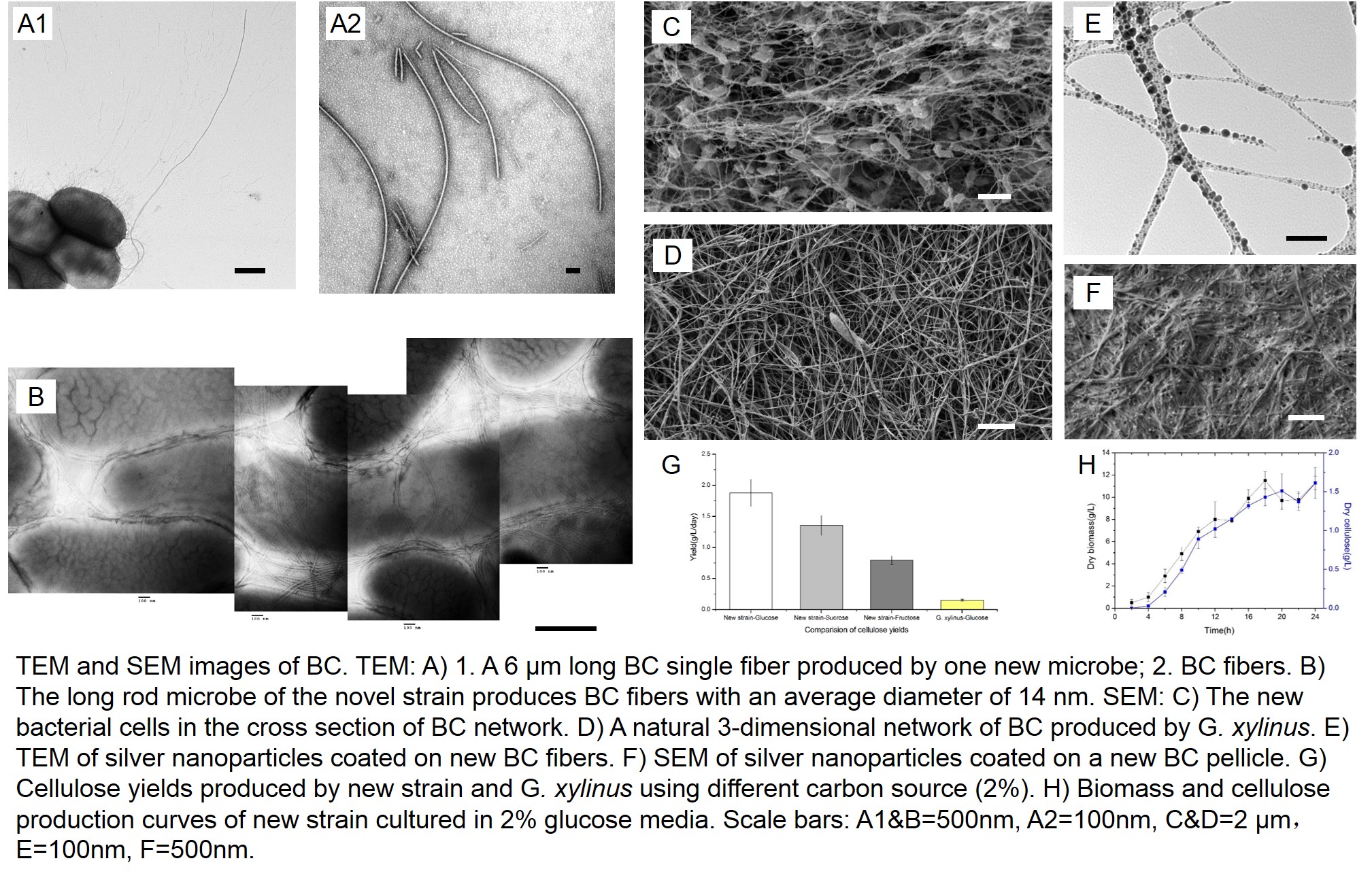
Results (Figure 1 and Figure 2) show the morphologies of bacteria and BC, as well as the nano-silver coated BC. The results demonstrate that: 1) under static culture conditions, the bacteria has a significantly higher BC productivity and production rate compared to G. xylinus; 2) glucose and sucrose are better carbon sources than fructose; 3) the BC produced by both strains of bacteria are chemically identical; 4) the purification with NaOH of new BC is simple and effective; 5) the BC fibers have a smaller diameter than the BC ribbons produced by G. xylinus, which indicates a higher specific surface area; and 6) the crystallinity of BC is higher than that of BC produced by G. xylinus. This novel strain of bacterium is also able to produce BC in a variety of sizes and shapes under oxygen limited environment, which highly simplifies the production process.
Conclusion: Using this novel strain of bacterium, we exploit the facultative nature of this bacterium for medical device developments. This novel strain can reduce the cost of BC production and accelerate the manufacturing process of wound dressings and antimicrobial wound healing products from this novel strain. This discovery has the potential of leading to a significant improvement in commercialization not only in soft tissue regeneration but also in all BC applications.
Dr. Richard Gardiner and UWO Biotron for providing the TEM instruments, training and guidance with sample preparations; Karen Nygard and UWO Biotron for providing the confocal workstation, fluorescence microscope and SEM instruments and training with instruments; Dr. Jian Liu and UWO Nanofabrication for supplying SEM and EDAX instruments and observations; Ying Li for AFM operations and XRD measurements; Helium Mak for the compression test; Dr. Charles Wu and the UWO Biotron for the element analysis; Monique Durr and UWO Biotron for the gas chromatography and ion chromatography; Lina Fu’s work was funded by the MITACS Accelerate Internship award and MITACS Accelerate Postdoc Fellow Fellowship. Funding for this work was provided by the Ontario Research Fund and Axcelon Biopolymers Corporation
References:
[1] Römling, U., & Galperin, M. Y. (2015). Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends in microbiology, 23(9), 545-557.
[2] Fu, L., Zhang, J., & Yang, G. (2013). Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydrate polymers, 92(2), 1432-1442.
[3] Klemm, D., Shumann, D., Kramer, F., HeBler, N., Hornung, M., Schumauder, H., et al. (2006). Nanocelluloses as innovative polymers in research and application. Advances in Polymer Science, 205, 49–96.
[4] Petersen, N., & Gatenholm, P. (2011). Bacterial cellulose–based materials and medical devices current state and perspectives. Applied Microbiology and Biotechnology, 91, 1277–1286.
[5] Joseph, G., Rowe, G. E., Margaritis, A., & Wan, W. (2003). Effects of polyacrylamide‐co‐acrylic acid on cellulose production by Acetobacter xylinum. Journal of Chemical Technology and Biotechnology, 78(9), 964-970.
[6] Wan, W., & Guhados, G. (2013). Nanosilver coated bacterial cellulose. U.S. Patent No. 8,367,089. Washington, DC: U.S. Patent and Trademark Office.