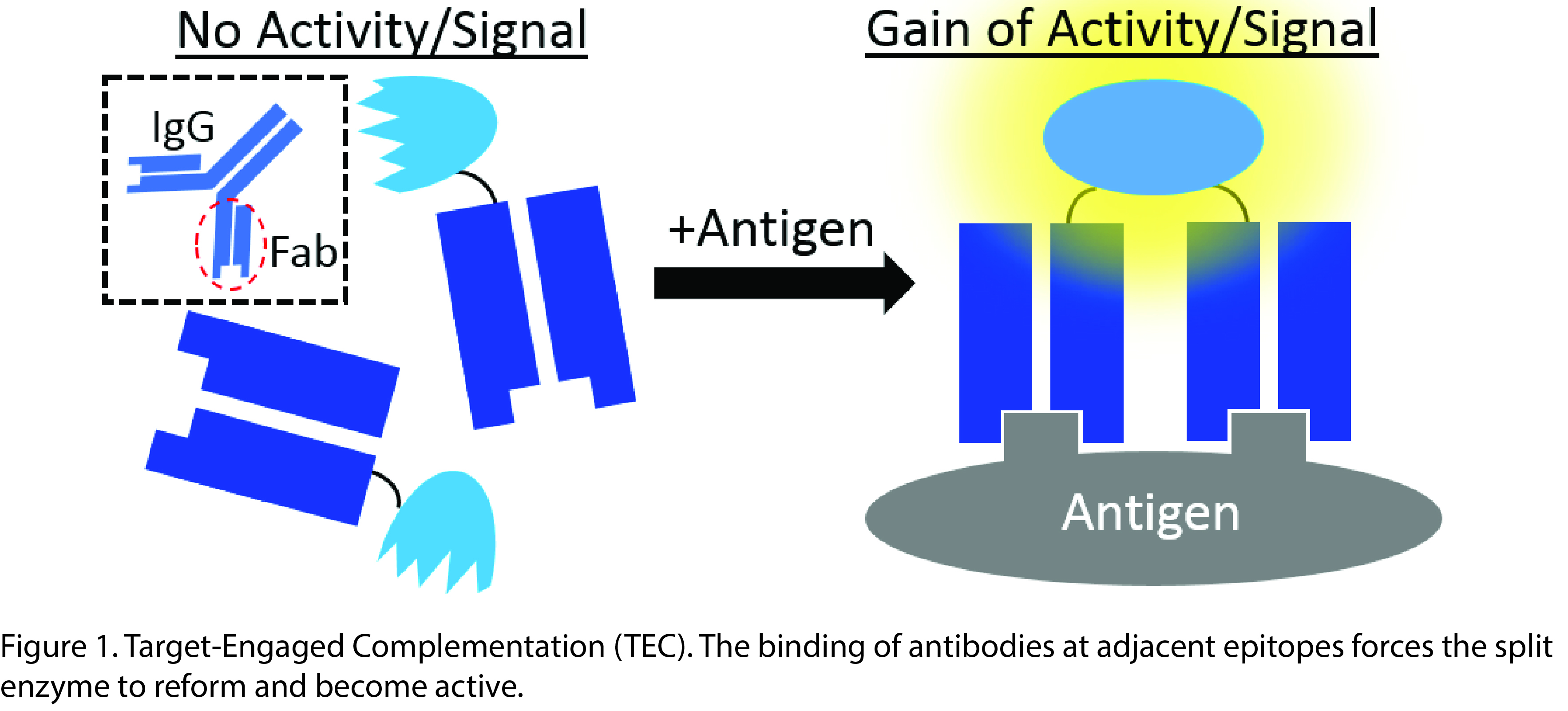
Diagnostic and imaging applications using antibodies require conjugating or labeling the antibody in order to obtain a measurable readout[1],[2]. As such, the readout signal is active prior to antibody-antigen binding, requiring significant effort to prevent high background and false signals. The same problem plagues therapeutic antibody-enzyme constructs – the enzyme is constantly active. We created a superior theranostic method in which the diagnostic signal or therapeutic activity is completely dormant until antibody binds to the target antigen. Our approach, termed Target Engaged Complementation (TEC), utilizes enzymes split into inactive components. Each half of the split enzyme is fused to an individual antibody fragment (Fab). The binding of the Fabs places the enzyme fragments into proximity where they complement to reform the active enzyme. Thus, activity is completely dependent on the antibody binding to the target antigen (Figure 1).
Materials and Methods: Potential non-overlapping antibody binding partners were identified in silico using reported crystal structures: trastuzumab (Herceptin ®) – 1N8Z; pertuzumab (Perjeta ®) – 1S78. The luciferase NanoLuc enzyme (Promega) was selected for diagnostic applications[3]. Fab-enzyme fusion proteins were produced recombinantly using the pF1k plasmid expressed in Shuffle T7 competent e. coli (NEB) and purified by affinity chromatography. Assays were performed by incubating live cells with Fab-enzyme fusions. Nano-Glo® (Promega) luciferase reagent was added and luminescence was measured on a M1000 Pro Tecan.
Results and Discussion: We selected the cancer biomarker HER2 as a target for an initial validation of the TEC concept and selected several antibody pairs from in silico measurements. The criteria for potential pairs are: 1) do not bind to overlapping epitopes and 2) bind so that the distance between pairs allows structural complementation of the split enzyme. We created fusion proteins from anti-HER2 Fabs and fragments from the small luciferase NanoLuc® which has been previously split into fragments that reconstitute activated enzyme following complementation (Figure 2)[4].
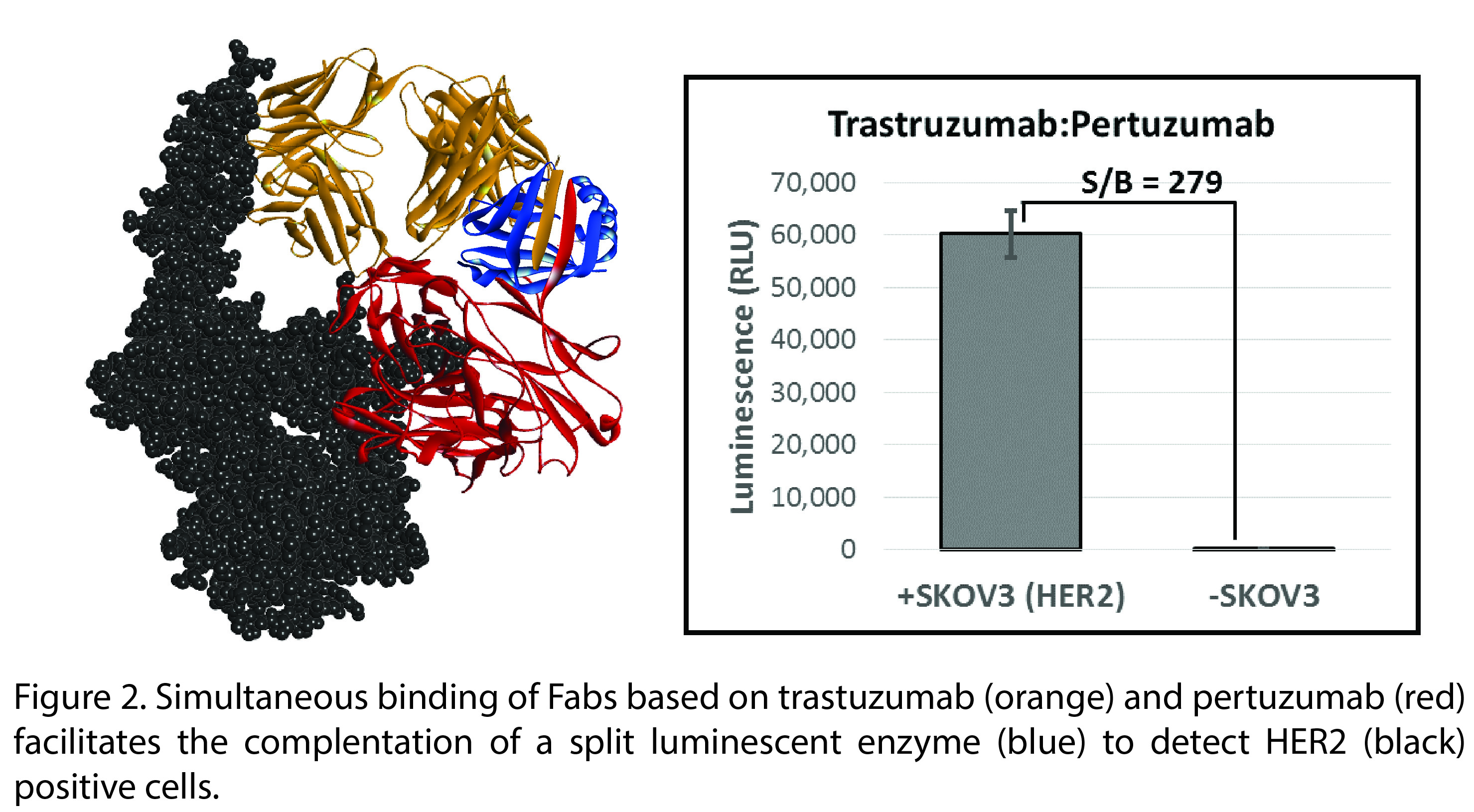
Lumiescence assays reveal the ability of these constructs to detect HER2 on the ovarian cancer cell line SKOV3 (Figure 2) with a signal to noise ratio ~300:1. In addition, TEC was effective in detecting the shed extracellular domain of HER2 in solution (data not shown). Neither assay required any washing steps to remove unbound antibody. As such, the specific interaction of antibody with antigen is sufficient to force enzyme complementation to detect an important clinical cancer biomarker.
Conclusions: In addition to diagnostics, TEC may be used for therapeutic applications. An original inception of targeted therapeutics involved an approach termed Antibody-Dependent Enzyme Prodrug Therapy (ADEPT), where a full enzyme was fused with an antibody and used to convert a prodrug[5]. The ability of the enzyme to continuously convert prodrug at the tumor site is extremely appealing, yet first compositions of ADEPT suffered from a constitutively active enzyme that activated the prodrug at off-target sites. TEC is an alternative approach that can potentially activate prodrugs specifically to the tumor site. Success has the field-shifting potential of creating a unique theranostic platform applicable to oncology and other clinically relevant areas.
References:
[1] Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, et al. Antibody validation. Biotechniques. 2010 Mar;48(3):197-209.
[2] Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry. 1971 1971/09/01;8(9):871-4.
[3] Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol. 2012 Nov 16;7(11):1848-57.
[4] Andrew S. Dixon LE, Mary Hall, Keith Wood, Monika Wood, Marie Schwinn, Brock F. Binkowski, Hicham Zegzouti, Nidhi Nath, Subhanjan Mondal, Said Goueli, Poncho Meisenheimer, Thomas Kirkland, James Unch, Dileep K. Pulukkunat, Matthew Robers, Melanie Dart, Thomas Machleidt inventor Promega Corporation, assignee. Activation of Bioluminescence by Structural Complementation patent 20140363375. 2014.
[5] Tietze LF, Schmuck K. Prodrugs for Targeted Tumor Therapies: Recent Developments in ADEPT, GDEPT and PMT. Curr Pharm Des. [Review]. 2011 Nov;17(32):3527-47.