Introduction: Worldwide, community-acquired pneumonia is a serious disease that is the leading cause of death in children less than five years of age[1]. Pneumococcal surface protein A (PspA) is a key virulence factor of Streptococcus pneumoniae. Polyanhydride nanoparticles comprised of copolymers of 1,6-bis(p-carboxyphenoxy) hexane (CPH) and 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) provide several benefits for vaccine delivery, including adjuvanticity, antigen stability, and sustained antigen release[2]. Upon release from nanoparticles, PspA maintains its primary and secondary structure including antigenicity[3]. Previous work has shown that encapsulation of a suboptimal dose of antigen into polyanhydride microparticles can induce a robust, long-lived immune response[4]. Reducing the antigen required in a vaccine may reduce vaccine production costs as well as vaccine shortages. Here, we expand upon previous work and demonstrate protection against lethal S. pneumoniae challenge following a single vaccination with a nanovaccine formulation containing a 25-fold lower dose of PspA than previously described[3].
Materials and Methods: CBA/CaHN mice were immunized subcutaneously with one of the following regimen: 1 µg PspA encapsulated into 50:50 CPTEG:CPH nanoparticles, 0.5 µg PspA encapsulated into 50:50 CPTEG:CPH nanoparticles plus 0.5 µg soluble PspA, 1 µg PspA plus Alum, 1 µg PspA alone, and saline. Serum samples were collected 21 days post-immunization (DPI) and analyzed via ELISA. Mice were challenged intravenously 24 DPI with 2500 CFUs of S. pneumoniae strain A66.1. Body temperature and body condition scores were recorded daily for the duration of the experiment. Moribund mice were removed from the study and at 14 days post-challenge surviving animals were euthanized.
Results and Discussion: Mice immunized once with PspA encapsulated into nanoparticles had significatly better survival rates (87.5%) over those immunized with soluble PspA alone (12%) (Fig. 1A). Although there was no difference in survival between the nanovaccine- and the PspA plus Alum-immunized mice, the mice that received the nanovaccine formulations had higher antibody titers (Fig. 1B). Additionally, no difference in survival or antibody titer was observed between the mice that received 100% PspA encapsulated into nanoparticles and those that received 50% of the PspA encapsulated into nanoparticles plus 50% as soluble PspA. Eliminating the need for a soluble bolous of PspA will improve the potential for the prolonged shelf life of the PspA-containing nanovaccine at room-temperature or above, based on the favorable thermal stability profile of polyanhydride nanoparticles[5].
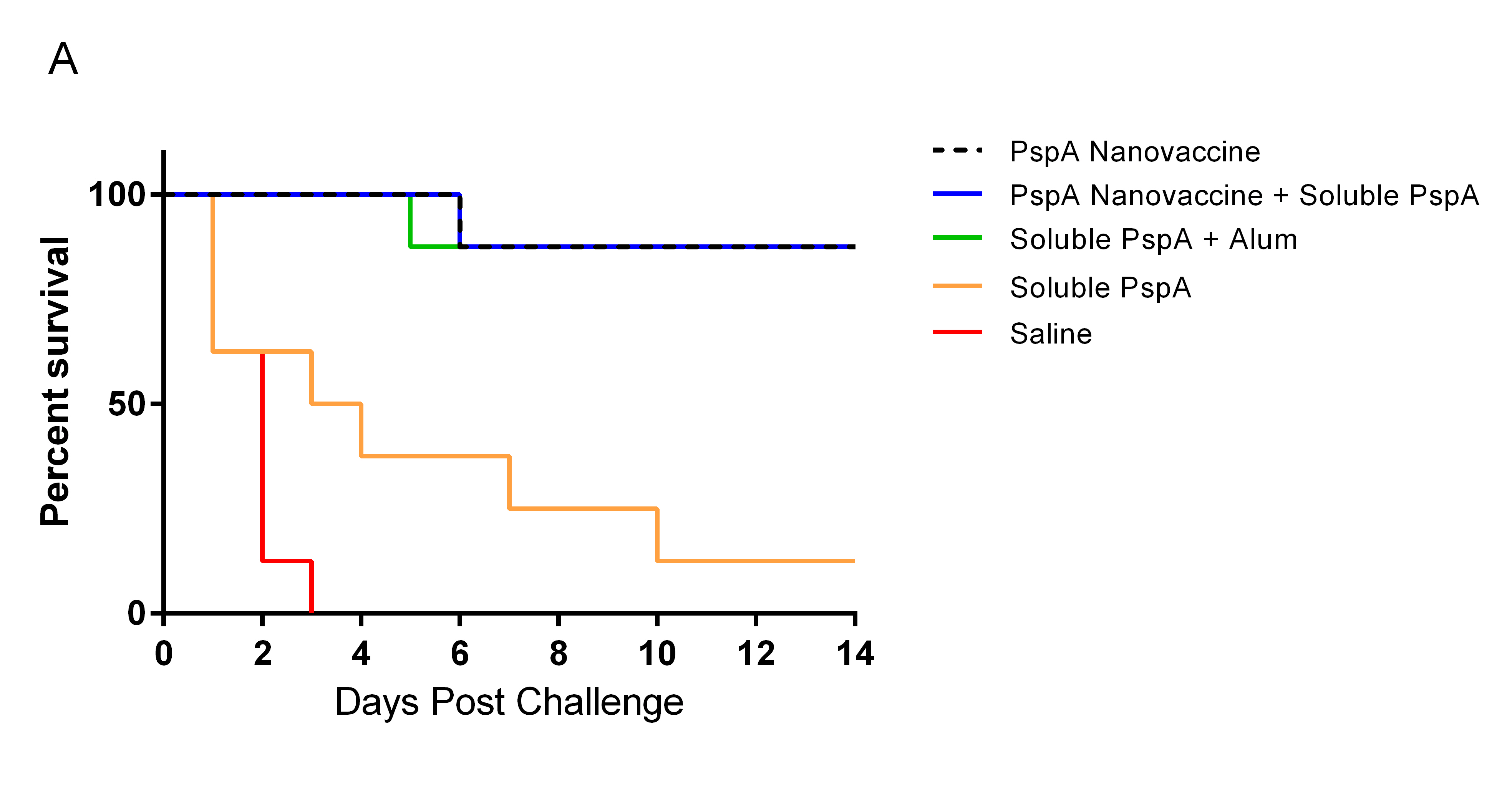
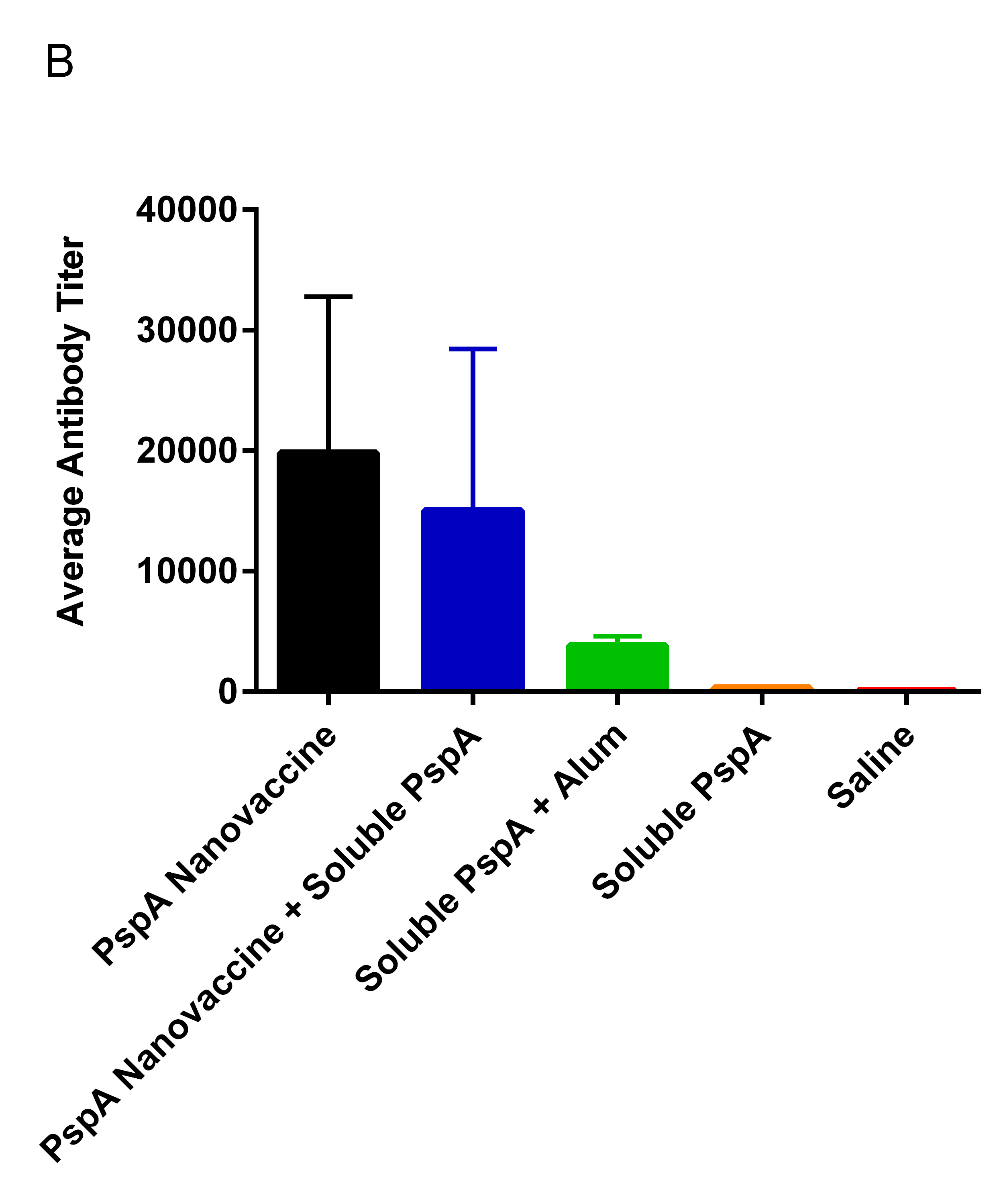
Conclusion: Similar to the use of Alum, immunization with PspA encapsulated into polyanhydride nanoparticles provided protection against lethal S. pneumoniae challenge but with much less tissue reactogenicity[6]. Both nanovaccine regimens also induced robust humoral immune responses that were comparable to that observed following immunization with a 25-fold higher dose of soluble PspA[3].
References:
[1] K. Stuckey-Schrock, B.L. Hayes, Am Fam Physician, 86 (2012) 661-667
[2] S.L. Haughney, K.A. Ross, P.M. Boggiatto, M.J. Wannemuehler, B. Narasimhan, Nanoscale, 6 (2014) 13770-13778
[3] S.L. Haughney, L.K. Petersen, M. Wannemuehler, B. Narasimhan, B. Bellaire, Acta Biomaterialia, 9 (2013) 8262-8271.
[4] L. Huntimer, J.H. Wilson Welder, K. Ross, B. Narasimhan, M. Wannemuehler, A. E. Ramer-Tait, J Biomed Mater Res Part B 101B (2013) 91-98
[5] L.K. Petersen, Y. Phanse, A.E. Ramer-Tait, M.J. Wannemuehler, B. Narasimhan, Mol. Pharmaceutics, 9 (2012) 874-882
[6] L. Huntimer, A. E. Ramer-Tait, L.K. Petersen, K.A. Ross, K.A. Walz, C. Wang, J. Hostetter, B. Narasimhan, M.J. Wannemuehler, Adv. Healthcare Mater. 2 (2013) 369-378