According to the International Diabetes Federation, 387M people are living with diabetes of which 46.3% are undiagnosed [1]. Uncontrolled diabetes can be a contraindication to dental implants [2]. It is estimated that 5% of dental implant failures of unknown causes and maybe associated with uncontrolled systemic disease. Consensus in literature is that hyperglycemia causes a delay in bone growth but the exact mechanisms are unknown. Ajami et al. believe that the delay occurs in the early stages of bone growth [3]. This study aims to identify the differences in normal and hyperglycemic bone formation at an early time point using high-resolution transmission electron microscopy.
Twelve, young (200-250grm) male Wistar rats were used for this study with six rats were assigned to the control group and the other six rats were intravenously injected with 65 mg/kg of streptozocin (STZ) to induce hyperglycemia, as previously described [3]. An osteotomy drill-hole model was used to make a 1.3mm defect in the diaphysis of the rat femurs. After five days, the femurs were removed, fixed in glutaraldehyde, dehydrated, and embedded prior to cutting into cross-sections for electron microscopy. Samples were prepared for transmission electron microscopy (TEM) using an ultramicrotome. The samples were stained with uranyl acetate and lead citrate before being coated with carbon. Selected area electron diffraction (SAED) and energy dispersive electron microscopy (EDS) were used to examine three areas of interest from both sample groups including areas of the old bone, the woven bone and the collagen fibrils near the woven bone.
Quan & Sone believe that less mineralized collagen fibrils would have a smaller diameter and a larger D-spacing than more mineralized collagen fibrils [4]. Therefore, if the hyperglycemic samples were less mineralized as we predict they would be, they should have a smaller diameter and larger D-spacing than the normal samples. However, there was not a significant difference between the groups suggesting no difference in mineralization levels within the collagen fibrils. SAED for both sample groups showed that the old bone had crystals orientated along the c-axis of the collagen fibrils, while the woven bone contained mostly amorphous or possibly nanocrystalline mineral, and the collagen fibrils surrounding the woven bone did not contain any detectable mineral.
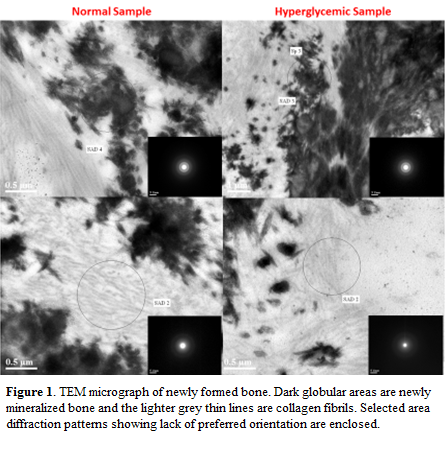
Additionally, EDS for both sample groups in the bone and woven bone showed calcium and phosphorous, whereas, the collagen fibrils near the woven bone did not appear to have calcium and phosphorous. Furthermore, electron energy loss spectroscopy (EELS) did not show calcium in the collagen fibrils. This data suggests there does not appear a difference in intrafibrillar mineralization in the new woven bone for both sample. Therefore, it does not appear that a disruption in intrafibrillar mineralization is the reason for delayed bone growth in hyperglycemia at the five day time point in rats. A more robust approach to identifying the same stage of mineralization in each group may be needed to identify any differences.
References:
[1] N. A. Mena, E. A. Sea, and S. Lucia, “IDF Diabetes Atlas 6th edn. 2014 update,” 2014
[2] H. Abdulwassie and P. J. Dhanrajani, “Diabetes mellitus and dental implants: a clinical study.,” Implant Dent., vol. 11, no. 1, pp. 83–86, 2002.
[3] E. Ajami, et al, “Bone healing and the effect of implant surface topography on osteoconduction in hyperglycemia.,” Acta Biomater., vol. 10, no. 1, pp. 394–405, Jan. 2014.
[4] B. D. Quan and E. D. Sone, “Structural changes in collagen fibrils across a mineralized interface revealed by cryo-TEM,” Bone, vol. 77, pp. 42–49, 2015.