Introduction: Bone is a hybrid nanocomposite of 70 wt% inorganic carbonated hydroxyapatite (CHA) nano-platelets and 30 wt% organic matrix, dominated by type-I collagen[1],[2]. Along with anionic non-collagenous proteins, small biomolecular citrates with strong negative charge, are also frequently found in bone (at 2 wt%; corresponding to >90% of the total citrate content in the body)[3]-[5]. However, the role of citric acid on bone biomineralization remains a subject of debate, though it is accepted that citrate plays prominent roles in bone anatomy, physiology, as well as orthopaedic biomaterials development[6],[7]. Here, we demonstrate that both nano intrafibrillar and macro interfibrillar mineralization of osteoid-like dense collagen (DC) gels are achieved by simply introducing citric acid during collagen gel fibrillogenesis and exposure to simulated body fluid (SBF).
Materials and Methods: Collagen gel aspiration-ejection was used to generate DC gels[8]. Concentrated citric acid solution (100 mM/L) was added to the neutralized collagen solution to a desired concentration during fibrillogenesis. After densification, DC gels were washed in distilled water and mineralized in SBF[9] at 37ºC.
The promotion of DC negative charge in the presence of citric acid was investigated by its capacity to bind to cationic dyes using methylene blue. Mineralization degree was quantitatively measured using a calcium assay kit. Intrafibrillar and interfibrillar mineralization extent from nano-to-macro scale was assessed using SEM, TEM, AFM, XRD, ATR-FTIR and Micro-CT.
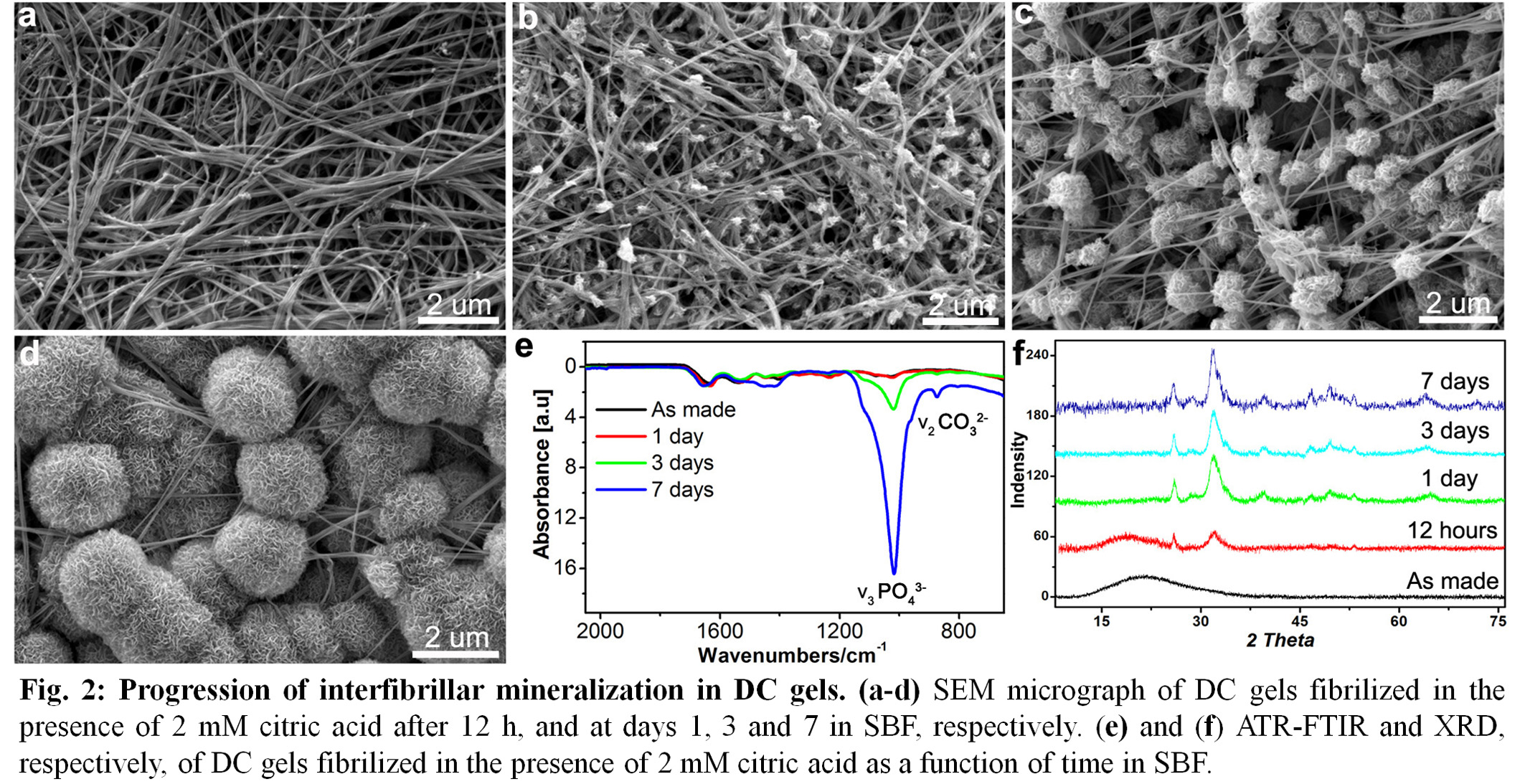
Results: In the presence of citric acid, nano intrafibrillar mineralization of collagen was induced within 12 h in SBF (Fig. 1). Extended exposure times to SBF, resulted in the interfibrillar mineralization of DC gels with the amorphous-to-crystalline transformation of CHA (Fig.2).
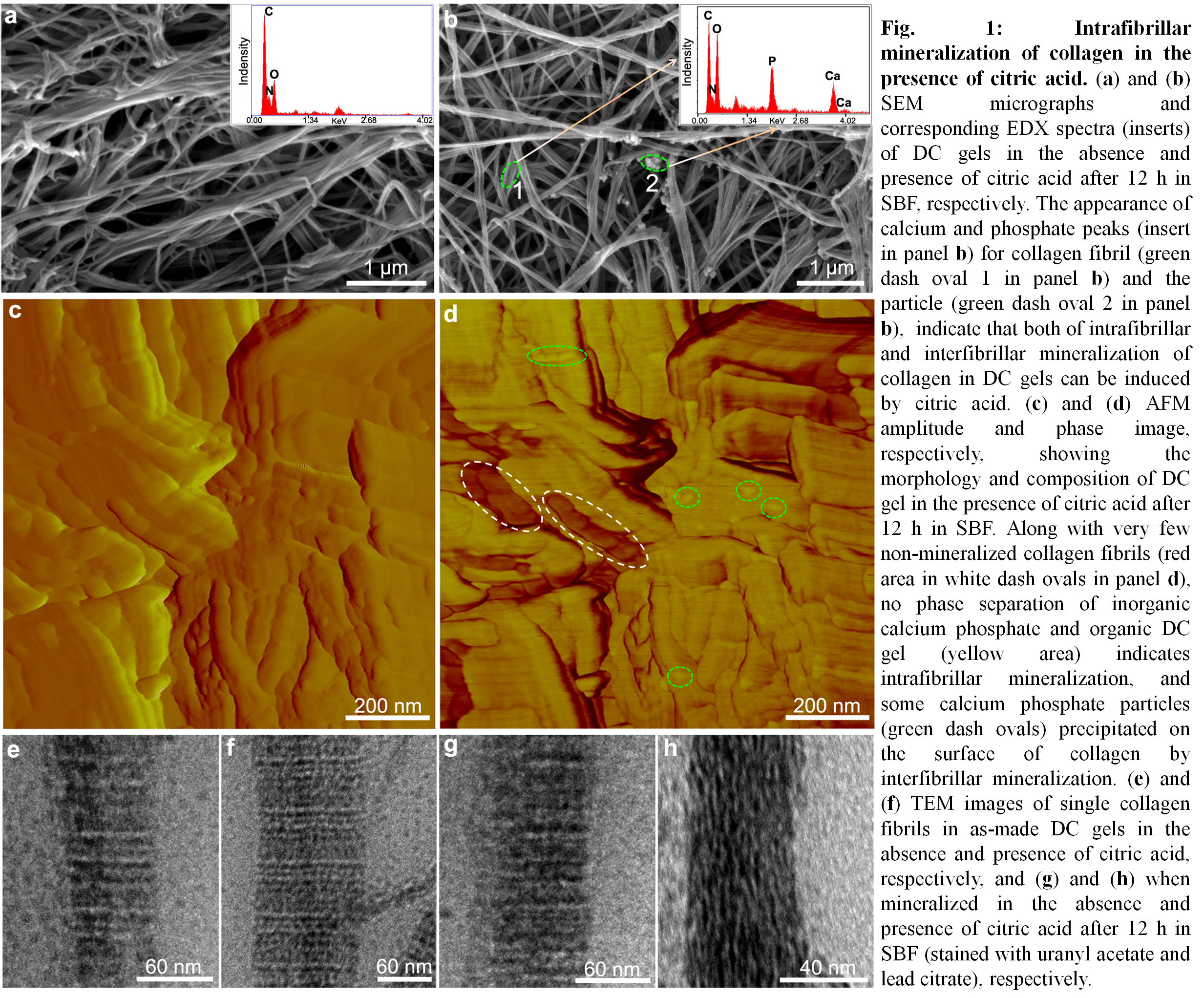
The negative charge of collagen fibrils was increased through citric acid binding that triggered calcium phosphate precipitation and extensive mineralization of DC gels (Fig. 3).
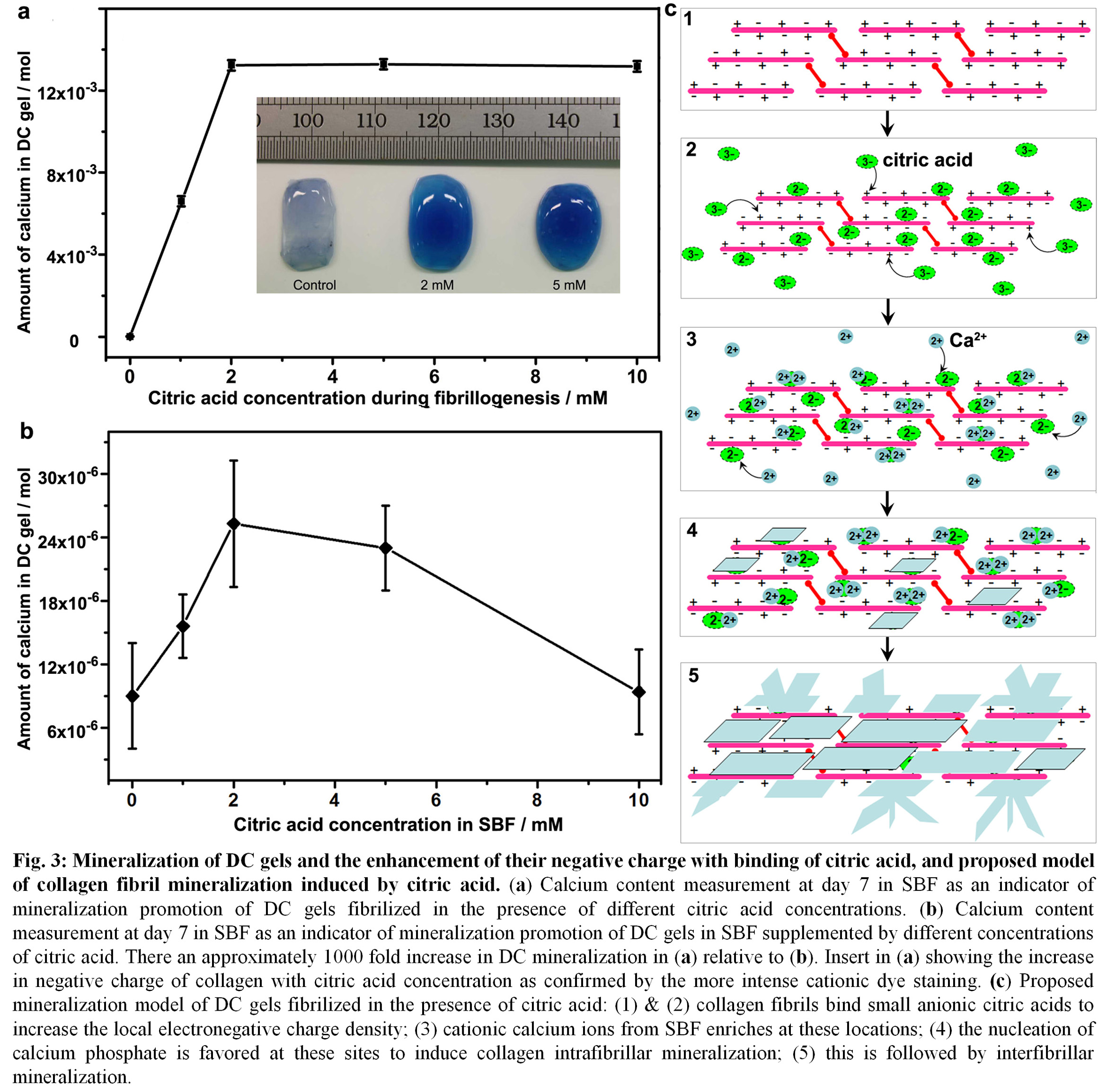
Discussion: Previous studies have only focused on the role of citric acid in forming CHA by simply adding these molecules to SBF or supersaturated solutions of calcium phosphate[10],[11]. This inhibits CHA formation due to the absorption of free ionic citric acid from solution that bind with calcium ions and block the binding of phosphate ions[12]. By excluding the inhibiting effects of free citric acid and its introduction into DC gels during fibrillogenesis, both intrafibrillar and interfibrillar mineralization were achieved. The promotion of DC gel negative charge resulted in its biomineralization attributable to not only shielding the cationic groups of collagen, but also adding two free anionic carboxyl groups of citric acid (Fig. 3).
Conclusions: These findings advance our understanding of the effects of citric acid on the biomineralization of collagen proteins and its role in bone formation, and provide insights into nucleation mechanisms of CHA in biomineralization.
Funding of NSERC, CFI, Quebec MEIE, MEDA and McGill University Faculty of Engineering Gerald Hatch Faculty Fellowship are gratefully acknowledged.
References:
[1] A. George & S. Ravindran, Nano Today 2010, 5, 254-266
[2] S. Pina, J. M. Oliveira & R. L. Reis, Adv. Mater. 2015, 27, 1143-1169
[3] S. Rhee & J. Tanaka, Biomaterials 1999, 20, 2155-2160.
[4] Y. Y. Hu, A. Rawal & K. Schmidt-Rohr, Proc. Natl. Acad. Sci. US A. 2010, 107, 22425-22429.
[5] E. Davies, K. H. Müller, W. C. Wong, C. J. Pickard, D. G. Reid, J. N. Skepper & M. J. Duer, Proc. Natl. Acad. Sci. USA 2014, 111, E1354-E1363
[6] R. T. Tran, J. Yang & G. A. Ameer, Annu. Rev. Mater. Res. 2015. 45, 277-310
[7] J. E. Barralet, M. Hofmann, L. M. Grover & U. Gbureck, Adv Mater. 2003, 15, 2091–2094.
[8] B. Marelli, C. Ghezzi, M. James-Bhasin & S. Nazhat. Biomaterials 2015, 37, 183-193.
[9] T. Kokubo & H. Takadama, Biomaterials 2006, 27, 2907-2915
[10] I. Yamaguchi, S. Iizuka, A. Osaka, H. Monma & J. Tanaka, Colloid Surface A, 2003, 214, 111-118
[11] J. L. Chen, B. Chu & B. S. Hsiao, J. Biomed. Mater. Res. A, 2006, 79A, 307-317
[12] W. H. Lee, C. Y. Loo, A. V. Zavgorodniy, M. Ghadiri & R. Rohanizadeh, RSC Adv., 2013, 3, 4040-4051.