Introduction: Vascular dysfunction can potentially be repaired by vasculogenic progenitor cells such as endothelial progenitor cells (EPCs)[1]. However, cell sources of EPCs are scarce. The number of circulating EPCs in the peripheral blood is low, and the number further decreases with the progression of vascular damage and with age in humans[2]. Thus, a derivation of endothelial-like cells with vasculogenic properties similar to EPCs from alternative sources would be in demand. It has been shown that human mesenchymal stem cells (hMSCs) transdifferentiate into endothelial-like cells with the induction of a biochemical cue, Vascular Endothelial Growth Factor (VEGF)[3],[4]. We hypothesize that hMSC transdifferentiate into endothelial-like cells with vasculogenic properties similar to EPCs with the induction of a physical and biochemical cue. In this study, the transdifferentiation of hMSCs into endothelial-like cells for vascular repair with induction of a physical cue and the vasculogenic properties of the trasndifferentiated endothelial-like cells were investigated.
Methods: The hMSCs from bone marrow were induced to transdifferentiate in vitro into endothelial-like cells through the provision of biochemical cue, VEGF and topographical features in the micro- and nanometer scale. To identify the most favorable topographies, we utilised a topography screening platform, the MultiARChitecture chip (MARC chip). The expression of endothelial cell (ECs) marker CD31 was quantified along morphometric analysis of transdifferentiated endothelial-like cells. Three best performing patterns were further evaluated in cell marker expression CD34, CD133, KDR and CD31 of the endothelial-like cells via flow cytometry. Furthermore, the functionality of endothelial-like cells was quantified by their tube-like structure formations in a Matrigel in vitro assay and by capillary formation in a Matrigel plug in vivo assay.
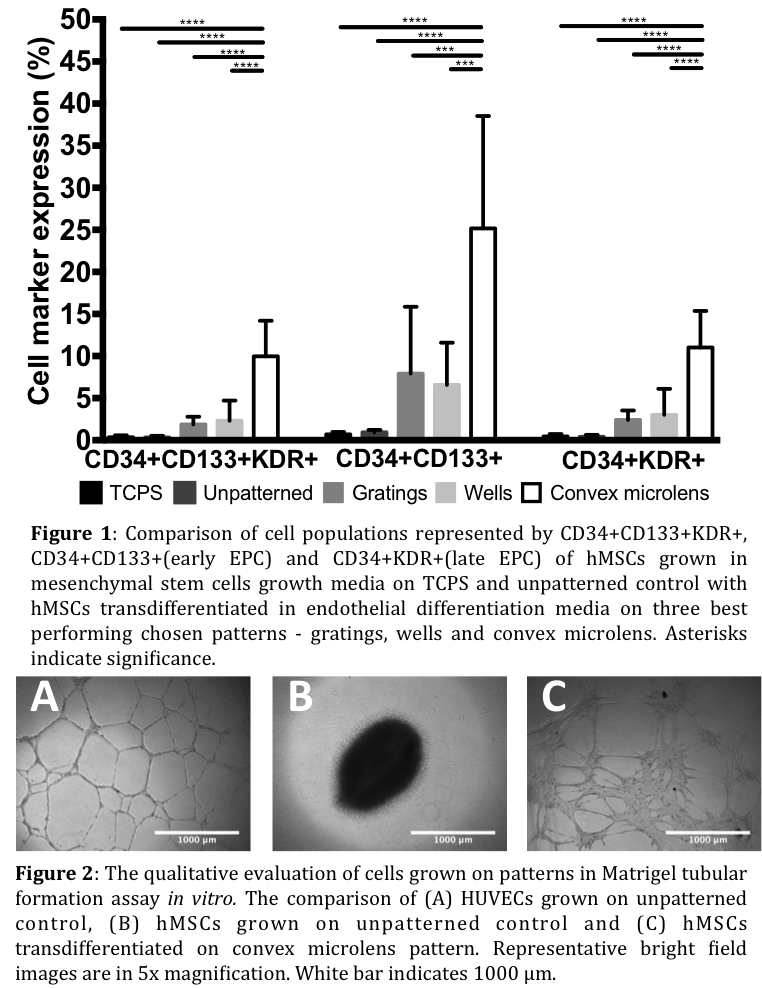
Results: Our study identified convex microlens pattern as the most influential topography that induced the endothelial transdifferentiation of hMSCs into endothelial-like cells with vasculogenic properties. Flow cytometric analysis showed up to 10% CD34+CD133+KDR+ positive cell populations with up to 25% CD34+CD133+ (similar to the characteristics of early EPCs) and up to 11% CD34+KDR+ (similar to the characteristics of late EPCs) populations (Figure 1). Endothelial-like cells and HUVECs formed tube-like structures on Matrigel in vitro, unlike undifferentiated hMSCs (Figure 2).
Furthermore, tube-like structure formation quantification of endothelial-like cells showed superiority in tube thickness, length of the tubes and number of branching points in comparison to the functional performance of HUVECs. Lastly, the Matrigel plugs with implanted endothelial-like cells showed higher number of capillary formation and inosculation over the plugs with undifferentiated hMSCs and HUVECs.
Conclusions: In conclusion, the results show that convex microlens topography influences the endothelial transdifferentiation of hMSCs into endothelial like cells that demonstrate cell marker expression and vasculogenic properties similar to EPCs. Furthermore, the capillary formation in vivo indicates the potential of transdifferentiated endothelial like cells to induce or participate directly in vasculogensis. These findings could be important to gain more understanding in vascular repair by cells with vasculogenic progenitor properties.
Mechanobiology Institute (MBI) R-714-007-006-271; Mechanobiology Institute (MBI) Scholarship
References:
[1] Calzi, S. L. et al. EPCs and pathological angiogenesis: When good cells go bad. Microvascular Research 79, 207–216 (2010)
[2] Leeper, N. J., Hunter, A. L. & Cooke, J. P. Stem Cell Therapy for Vascular Regeneration: Adult, Embryonic, and Induced Pluripotent Stem Cells. Circulation 122, 517–526 (2010)
[3] Oswald, J. et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22, 377–384 (2004)
[4] Chen, M.-Y., Lie, P.-C., Li, Z.-L. & Wei, X. Endothelial differentiation ofWharton’s jelly–derived mesenchymal stem cells in comparison with bone marrow–derived mesenchymal stem cells. Experimental Hematology 37, 629–640 (2009)