Introduction: MSC functions and cell fate have been affected by its microenvironments, including chemical environment (soluble and insoluble factors[1], surface chemistry[2] etc.) and physical environment (matrix elasticity, mechanical force[3]). The selection of matrix would likely exert important influence on MSC recruitment, attachment, condensation, proliferation that may ultimately affect the final fate of the MSCs.
Materials and Methods: Porous hydroxyapatite coatings were prepared through a novel liquid precursor plasma spraying process[4] and infiltrated with the collagen and synthetic hydrogel, respectively. Suspension of MSCs was seeded on HA-collagen and HA-synthetic hydrogel substrate surfaces, and MSC differentiation toward the osteoblastic and chondrocytic lineages were systematically compared as a results of the residing matrix. The effect of the presence of the inorganic substrates (Ti and HA) on MSC functions and cell fate was also examined in terms of adhesion, cytoskeleton organization, migration and differentiation.
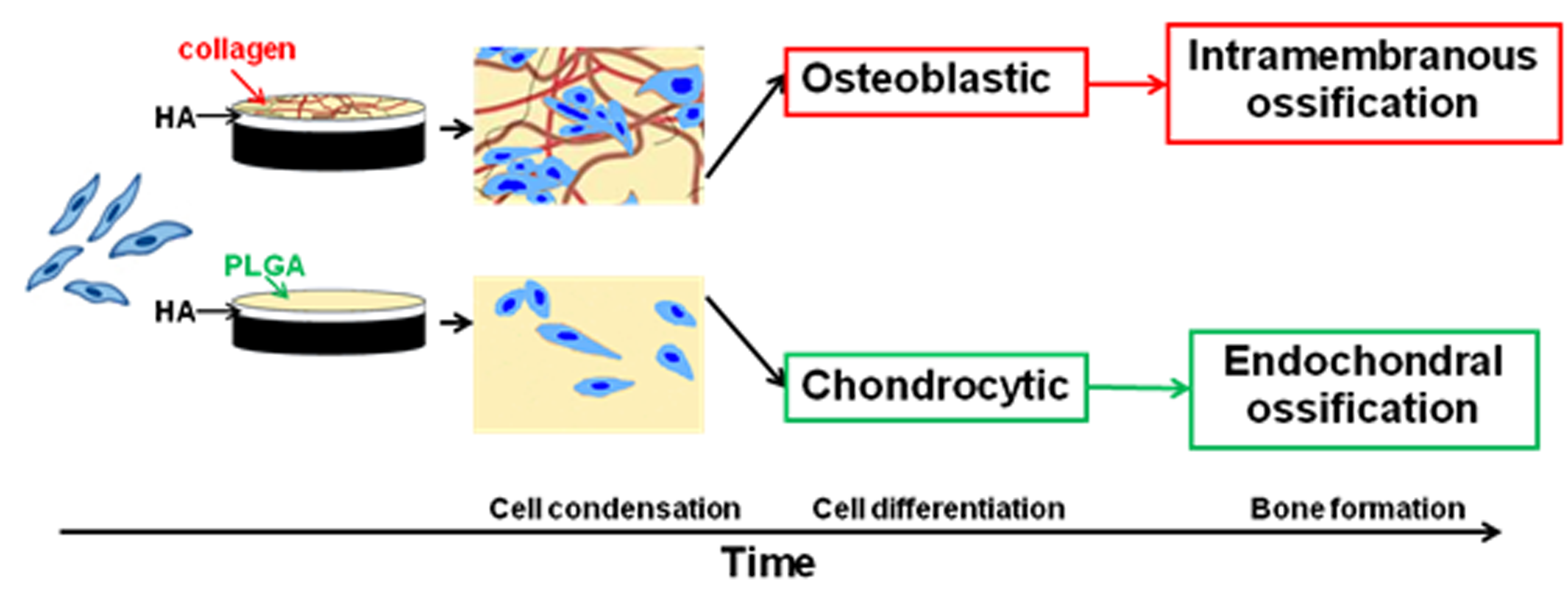
Result and Discussion: The present study reports that the matrix itself has a critical regulating effect on the MSC differentiation lineage and the selection of the bone formation mode. The HA-collagen group samples stimulated MSC differentiation into the osteoblastic lineage and showed inhibitory effect on chondrogenesis, as evidence by the up-regulation and strong upward trend of ALP, OPN and OCN expressions. In contrast, the HA-synthetic hydrogel group samples promoted MSC differentiation into the chondrocytic lineage as evidenced by the up-regulation and strong upward trend of Sox9, Col II and Col X. The HA-collagen/BMP samples also exhibited a straightforward intramembranous bone formation mode at ectopic sites, whereas the HA-synthetic hydrogel/BMP ones demonstrated an endochondral bone formation mode. Furthermore, The presence of the inorganic substrate likely provided suitable physical microenvironment that were beneficial for MSC cytoskeleton organization and migration, which are critical to drive the MSC differentiation into the osteoblastic lineage.
Conclusion: Cell matrix interaction is a critical factor that affects the biological responses of MSCs. The present study reports that the matrix has a critical regulating effect on MSC differentiation and the subsequent bone formation modes. A simply combined HA-collagen matrix stimulates the MSC differentiation into the osteoblastic lineage and leads to a straightforward intramembranous bone formation mode, in contrast to the chondrocytic differentiation and endochondral mode observed on HA-synthetic hydrogel matrix. Furthermore, the presence of the inorganic substrate, in particular HA, played an indispensable role for collagen self-assembly and helped to provide a suitable physical environment for MSC actin cytoskeleton organization, migration which are critical for maintaining osteogenic differentiation.
Fig. 1. Road maps of matrix directed MSC differentiation and bone formation mode.
References:
[1] VH. Fan, et al., Stem Cells, 2007, 25, 1241
[2] DSW. Benoit, et al., Nature materials, 2008, 7, 816
[3] S. Even-Ram, et al., Cell, 2006, 126, 645
[4] J. He, et al., JBMRA, 2012; 100A: 1706-1715