Introduction: We developed poly(vanillin oxalate) (PVO) as a novel antioxidant polymer, which is able to scavenge H2O2 and exert anti-inflammatory and anti-apoptotic activity[1]. Vanillin is a major component of natural flavoring agent vanilla and is known to have potent antioxidant and anti-inflammatory activities. PVO was designed to covalently incorporate vanillin via acid-cleavable acetal linkages as well as H2O2-responsive peroxalate ester bonds. In chemical terms, peroxalate ester is rapidly oxidized by H2O2 to generate high energy intermediate dioxetanedione, which instantaneously decomposes into CO2[2]. We therefore hypothesized that peroxalate esters in the backbone of PVO reacts with H2O2 to generate CO2 and H2O2-triggered CO2 bubble-generation endows PVO nanoparticles with echogenicity, allowing ultrasound imaging. In this work, we report the potential of PVO nanoparticles as ultrasound imaging agents and therapeutics for ischemia-reperfusion (I/R) injury.
Materials and Methods: PVO nanoparticles of a mean size of 400 nm were developed using single emulsion method. Hepatic I/R of mice was induced by clamping the main trunk of hepatic artery and portal vein for 1 h. Immediately before reperfusion, PVO nanoparticles were administrated. Ultrasound backscatter mode images were collected with Sonix TOUCH with L14-5/38 transducer for 14 MHz. Anti-inflammatory and anti-apoptotic activity of PVO nanoparticles were also evaluated.
Results and Discussion: Ultrasound imaging of hepatic I/R injury with PVO nanoparticles. PVO nanoparticles could not enhance the ultrasound images of healthy liver, suggesting that PVO nanoparticles under normal physiological conditions could not generate bubbles necessary of the enhancement of ultrasound intensity. Immediately after injection of PVO nanoparticles to mice undergoing hepatic I/R injury, significantly enhanced ultrasound images were observed with hepatic I/R injury from 15 min after administration of PVO nanoparticles. The enhanced contrast lasted up to 50 min.
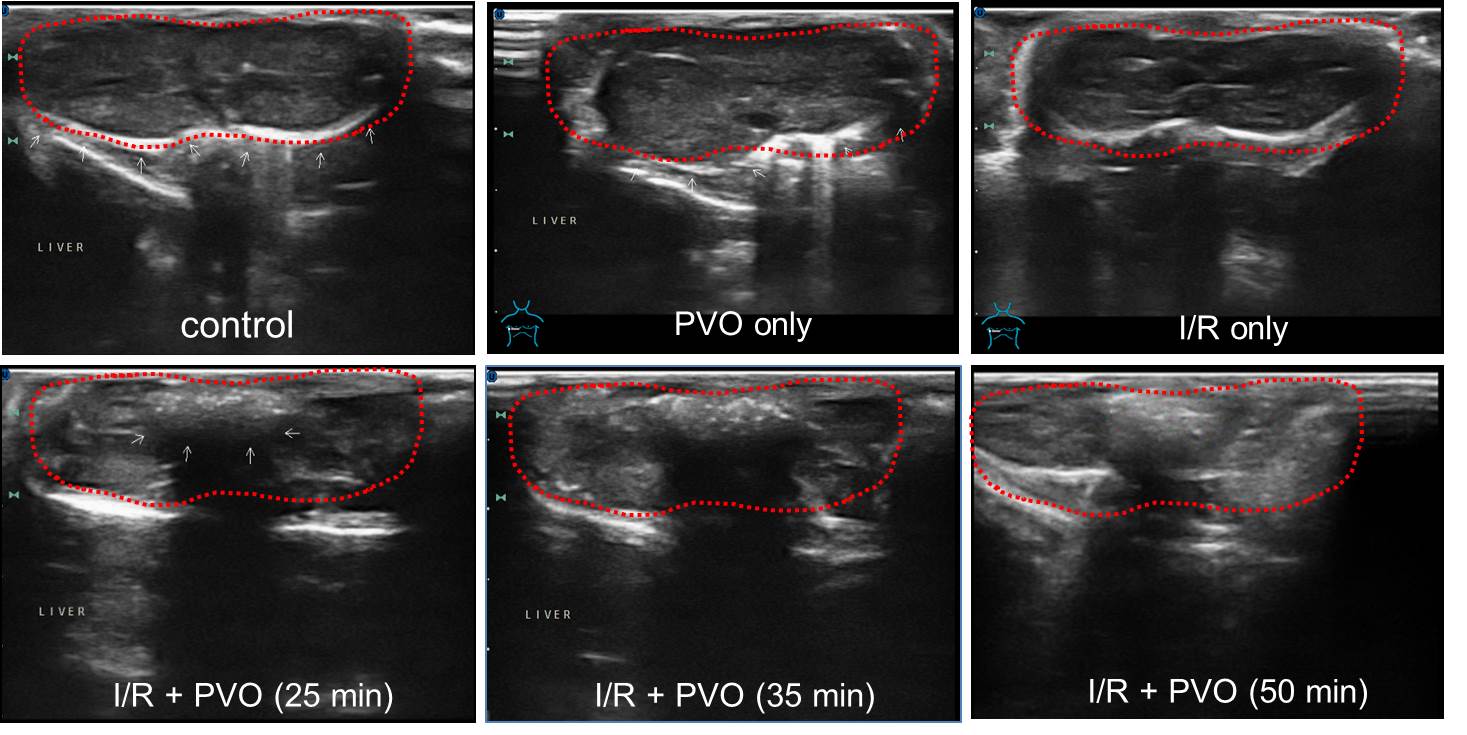
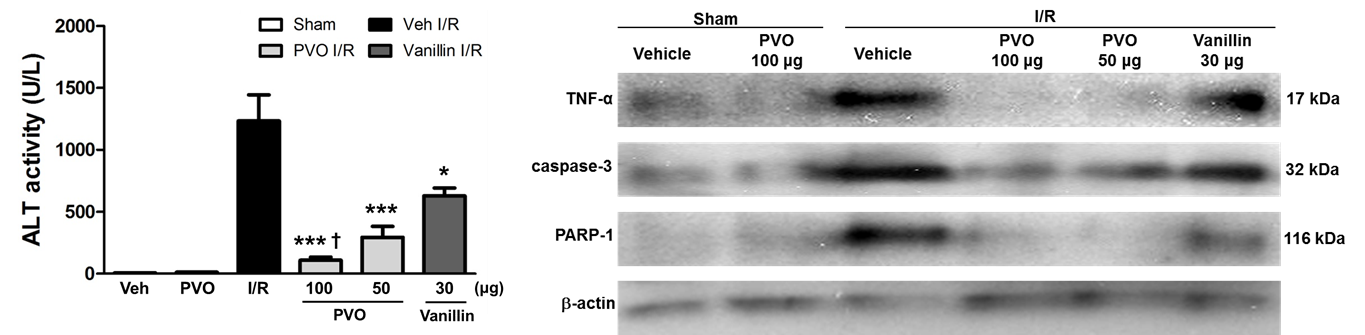
Therapeutic effects of PVO nanoparticles in hepartic I/R injury. Reperfusion into ischemic liver induced severe hepatic damages as evidenced by the remarkable increase in the level of ALT(alanine transaminase). PVO nanoparticles showed dose-dependent protective effects on hepatic I/R injury. A single dose of PVO nanoparticles significantly reduced the activity of ALT. Vanillin at a dose of 30 mg, which is equivalent to 100 mg of PVO nanoparticles also showed significant effects on the reduction of ALT level, but less potent protective effects compared to PVO nanoparticles. I/R injury induced over-expression of TNF-a. Vanillin had minimal inhibitory effects on the expression of TNF-a, but PVO nanoparticles exerted significant inhibitory effects on the expression of TNF-a. PVO nanoparticles also exerted anti-apopotic activity by suppressing the expression of caspase-3 and PARP-1.
Conclusions: PVO nanoparticles generated CO2 bubbles from H2O2-mediated oxidation of peroxalate esters and simultaneously released antioxidant vanillin. A single dose of PVO nanoparticles significantly enhanced ultrasound contrast in the site of hepatic I/R and remarkably inhibited the liver damages. We anticipate that PVO nanoparticles hold tremendous potential as a theranostic agent for simultaneous ultrasound imaging and therapy of H2O2-associated diseases.
References:
[1] Kwon, J.; Kim, J.; Park, S.; Khang, G.; Kang, P. M.; Lee, D., Inflammation-Responsive Antioxidant Nanoparticles Based on a Polymeric Prodrug of Vanillin. Biomacromolecules 2013, 14 (5), 1618-1626.
[2] Lee, D.; Bae, S.; Hong, D.; Lim, H.; Yoon, J. H.; Hwang, O.; Park, S.; Ke, Q.; Khang, G.; Kang, P. M., H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Scientific Reports 2013, 3.