Introduction: Articular cartilage has a limited ability to heal. Current surgical procedures to repair cartilage result in the formation of fibrocartilage instead of hyaline cartilage. The presence of fibrocartilage may suggest deficient bioactivity to promote the chondrocyte phenotype. Glycosaminoglycans (GAGs) have been shown to interact and maintain the bioactivity of growth factors due to their level and spatial distribution of sulfate groups[1]. Cellulose sulfate, a semi-synthetic derivative of cellulose, is a sulfated polysaccharide with structural similarity to GAGs and has yet to be explored for cartilage repair. It is a cost-effective alternative to the use of naturally occurring GAGs for cartilage repair applications. This study evaluated the effect of varying the degree of sulfation in cellulose sulfate on human mesenchymal stem cell (MSC) chondrogenesis and its effect on maintaining the bioactivity of transforming growth factor-β3 (TGF-β3), a potent driver of chondrogenesis, in comparison to other sulfated GAGs.
Materials and Methods: Cellulose sulfate was combined with bovine gelatin, electrospun to form fibrous scaffolds and characterized for fiber dimension, interfiber spacing, and interactions with TGF-β3. Interactions with TGF-β3 were additionally evaluated in solutions of cellulose sulfate. Partially sulfated cellulose (pSC) and fully sulfated cellulose (NaCS) were evaluated at 0.1% and 5% dry weight of the scaffold. Gelatin (Gel) with no cellulose sulfate was used as a control. MSCs were seeded onto the scaffold and chondrogenesis was evaluated after 56 days through the production of collagen type 2, cell morphology, and gene expression of chondrogenic markers.
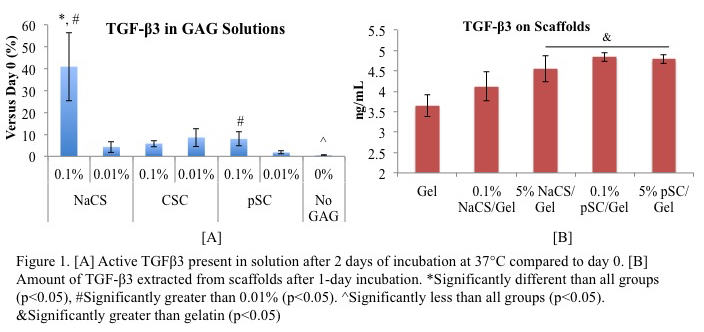
Results and Discussion: NaCS had significantly higher amounts of complexed TGF-β3 than chondroitin 6-sulfate (CSC) and pSC in solution (Figure 1A) and increasing the cellulose sulfate on the scaffold bound more TGF-β3 (Figure 1B).
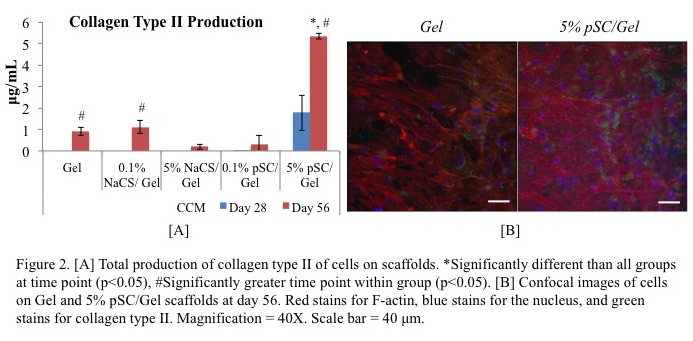
Yet, MSCs on pSC-gel scaffolds had the highest production of collagen type II (Figure 2A & 2B) and greatest gene expression for chondrocyte markers (aggrecan, collagen type II, chondroadherin, and Sox9).
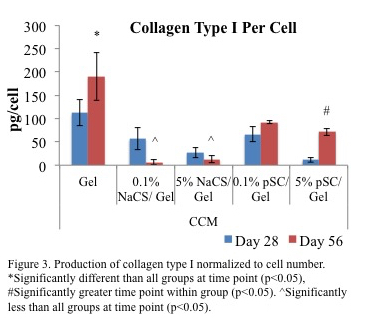
On scaffolds with cellulose sulfate, cells had a lower production of collagen type I per cell, a protein found in fibrocartilage, compared to gelatin alone (Figure 3).
The cells on the scaffolds had lower gene expression of collagens type X and I as indicators of hypertrophic chondrocytes and MSCs, respectively, when compared to cells in MSC chondrogenic control cultures.
Conclusion: The findings suggest that pSC-gel scaffolds, having partial sulfation, may support a more homogeneous cartilage tissue formation. Future studies will understand the interactions of the growth factors and GAGs to improve scaffold design and further promote tissue formation.
National Science Foundation; Musculoskeletal Transplant Foundation
References:
[1] Gama C, Tully S, Sotogaku N, Clark P, Rawat M, et al. (2006) Sulfation Patterns of Glycosaminoglycans Encode Molecular Recognition and Activity. Nature Chemical Biology 2: 467-473.