Abstract: An ideal tissue-engineering scaffold should resemble the ECM both in nanofiberous structure and components. Electrospinnig is a valuable technique to crate nanofiberous mats. But these electrospoun structures are very compact with small pore size which inhibits cell infiltration. In this work we performed a new technique in the use of electrospinning method to fabricate 3D highly porous structure with enhanced pore size compared to conventional compact elctrospoun mats. Morphological properties, pore size and porosity and enhanced cellular behaviour on the new-designed structure is verified and compared to those of conventional electrospoun structure.
Introduction: Ideal tissue engineering scaffolds should be capable of closely mimicking the topographies and spatial structures of native extracellular matrices (ECMs) to facilitate cells to grow and differentiate following the patterns similar to that found in native tissues and organs. Morphologies of ECMs vary according to functions of target tissues and cell types in the tissues. It has been reported that cells cultured on flat 2D substrates may differ considerably in morphology and differentiation pattern from those cultured in more physiological 3D environments [1]. In recent years, electrospinning has gained widespread interest as a potential tissue-engineering scaffolding technique because of the resultant ECM-like structure and has been discussed in detail in many studies. Apart from their clear advantages and extensive use, electrospun scaffolds encounter some practical limitations, [2] such as small pore size and porosity which leads to scarce cell infiltration which in turn makes the cells to spread on the surface and develop only into flat shape. But, the functions and differentiation of many flattened cells could not resemble the native stereoscopic cells. Increased porosity and pore size facilitate cell seeding and diffusion throughout the scaffolds, which is associated with increased vascularization, bone ingrowth, and osteointegration. In this work, we introduce a new 3D patterned nanofiberous structure made by the use of electrospinning/electrospraying technique that overcomes the above limitations.
Materials and method: polycaprolactone was purchased from Sigma company. Acetic acid and formic acid, were purchased from Merck company.
Prepared PCL solution with optimized viscosity suitable to fabricate our favourable structure, was poured in 5 ml syringe and put in electrospinning devise by the applied voltage of 20 Kv and injection rate of 0.5 ml/min. In this method, collector was covered with a metal mesh. [3]
Results and Discussion: SEM micrographs of prepared scaffolds are illustrated in fig 1. Compact microstructure of conventional electrospoun mat is shown compared to porous structure fabricated by our modified design.Porosity and pore size of scaffolds were also obtained and summarized in table 1.
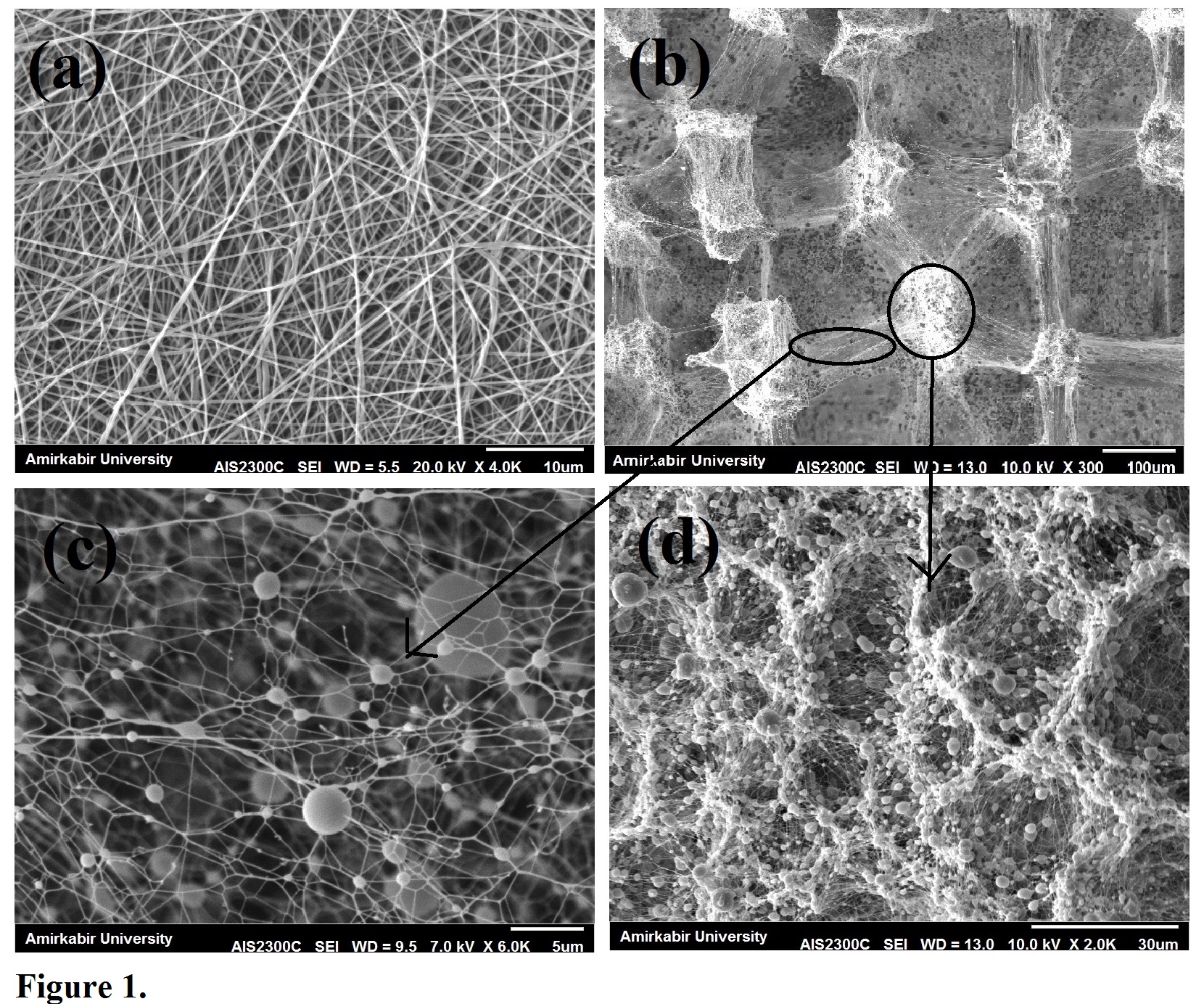
Figure 1. SEM micrographs of prepared Patterned 3D (b-d) and conventional 2D (a) electrospoun scaffolds.

Figure 2. illustrates the cell morphology and spread over the fabricated scaffolds. As it is obvious, cell attachment and spread is enhanced on the new-designed structure than conventional electrospoun mats. In addition, cells on the new designed 3D scaffolds try to make bridges over the large pores (pores with the size of about 100 μm) and form 3D shape cells instead of flattened on the surface and form 2D cells in conventional electrospoun mats.
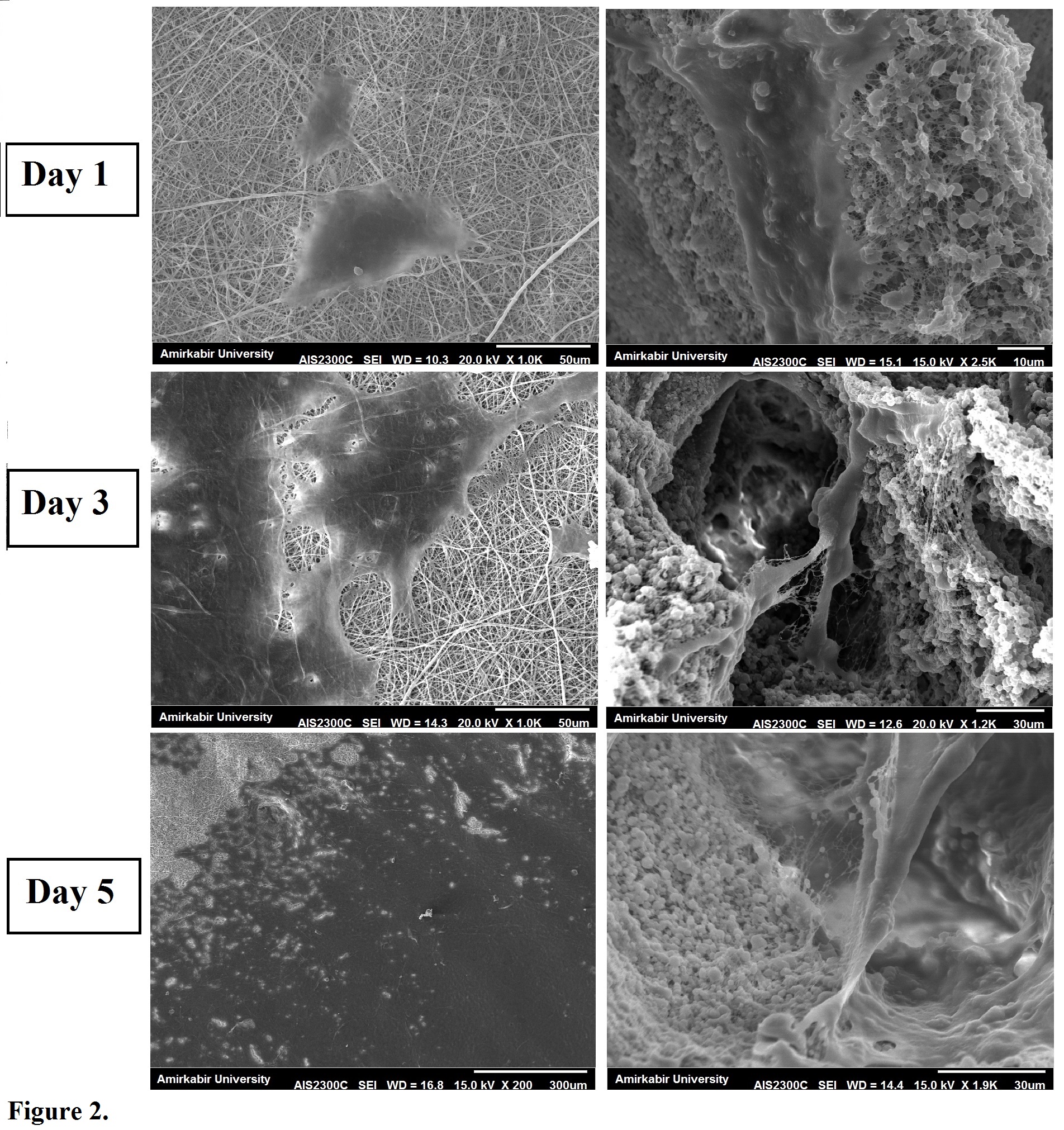
Figure 2. cell morphology on the prepared scaffolds. first column: conventional 2D scaffold, second column: prepared 3D patterned nanofiber/microparticle scaffold.
Conclusions: As the porosity and pore size in electrospinning technique is a challenging issue we demonstrated a new way to enhance these parameters in nanofiberous structure fabricated bypatterned electrospinning/electrospraying technique. This claim is confirmed by SEM micrographs and also porosity and pore size measurement. Enhanced cellular behavior is also illustrated by SEM images.
References:
[1] S. Cai, H. Xu, Q. Jiang, “Novel 3D Electrospun Scaffolds with Fibers Oriented Randomly and Evenly in Three Dimensions to Closely Mimic the Unique Architectures of Extracellular Matrices in Soft Tissues: Fabrication and Mechanism Study ”Langmuir 2013, 29, 2311−2318
[2] S. Khorshidi , A. Solouk, H. Mirzadeh, s Mazinani, JM Lagaron, S Sharifi, S Ramakrishna. “A review of key challenges of electrospun scaffoldsfor tissue-engineering applications”. J Tissue Eng Regen Med 2014,
[3] F. Hejazi, H. Mirzadeh, “Novel 3D scaffold with enhanced cell spread, proliferation and differentiation for bone tissue regeneration by simultaneous electrospinning and electrospraying, Part (A): Patterned structure, journal of biomedical material research, Part A, 2015