Introduction: To better guide the formation of functional tissues, it is preferable for the scaffolds to recapitulate the key physicochemical features of native extracellular matrix (ECM). In recognition of the multiscale fiber systems of ECM, it would be beneficial to fabricate the fiber scaffolds with a similar dimension and morphology. Electrospinning, a high voltage-driven spinning technology, has received a great attention in last a few years due to its capability of fabricating fibers in micron/submicron range[1],[2]. However, the poor mechanical properties and small interfiber pore sizes become the obstacles for their further utility in tissue engineering. In this regard, the current study was focused on the design of novel hybrid fiber scaffolds composed of prototyping microfibers (5-20 mm) and electrospun submicron fibers (200-600 nm) and the exploration of their use for bone tissue formation.
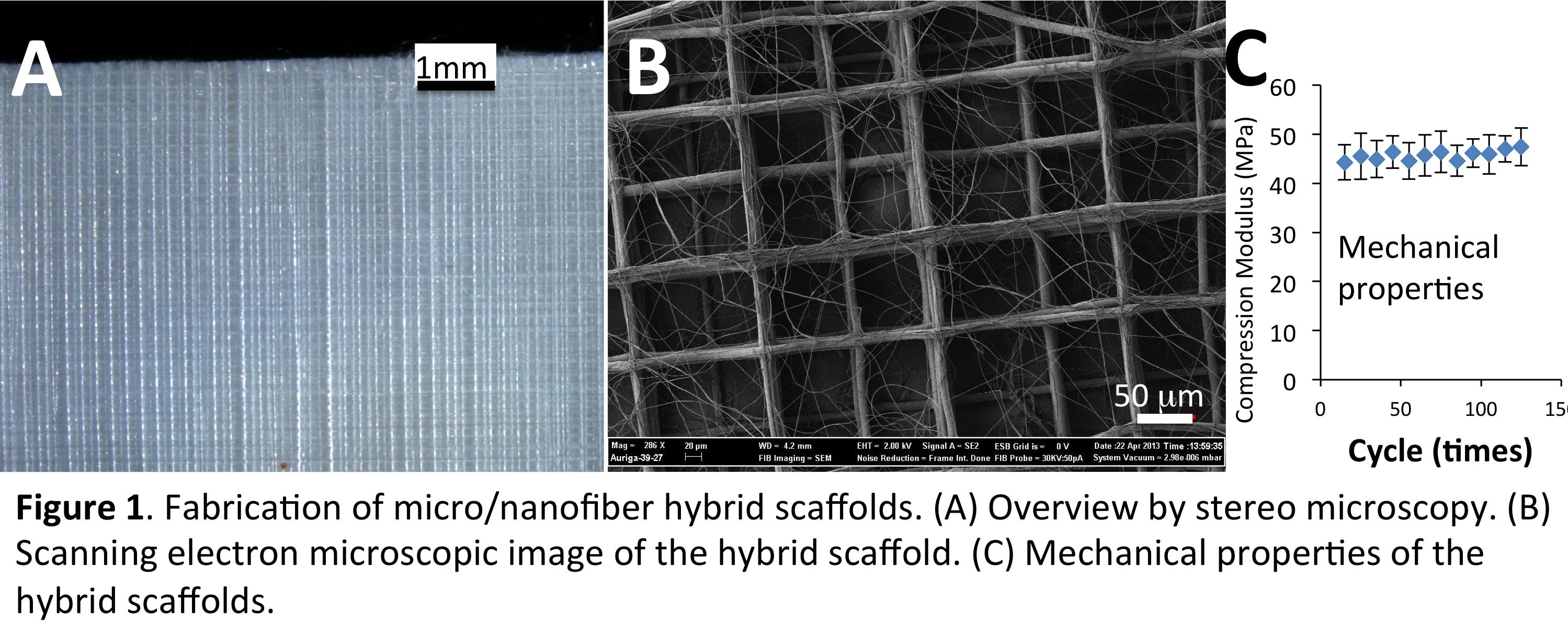
Materials and Methods: Fabrication of micro/nanofiber scaffolds: With the same setup while manipulating the electrospinning parameters, micro/nanofiber scaffolds composed of well-controlled polycaprolactone (PCL) microfiber grids (f=20 mm and interfiber distance=200 mm) sandwiched with random PCL/collagen nanofibers (200-600nm in diameter) were layer-by-layer fabricated for 20 layers.
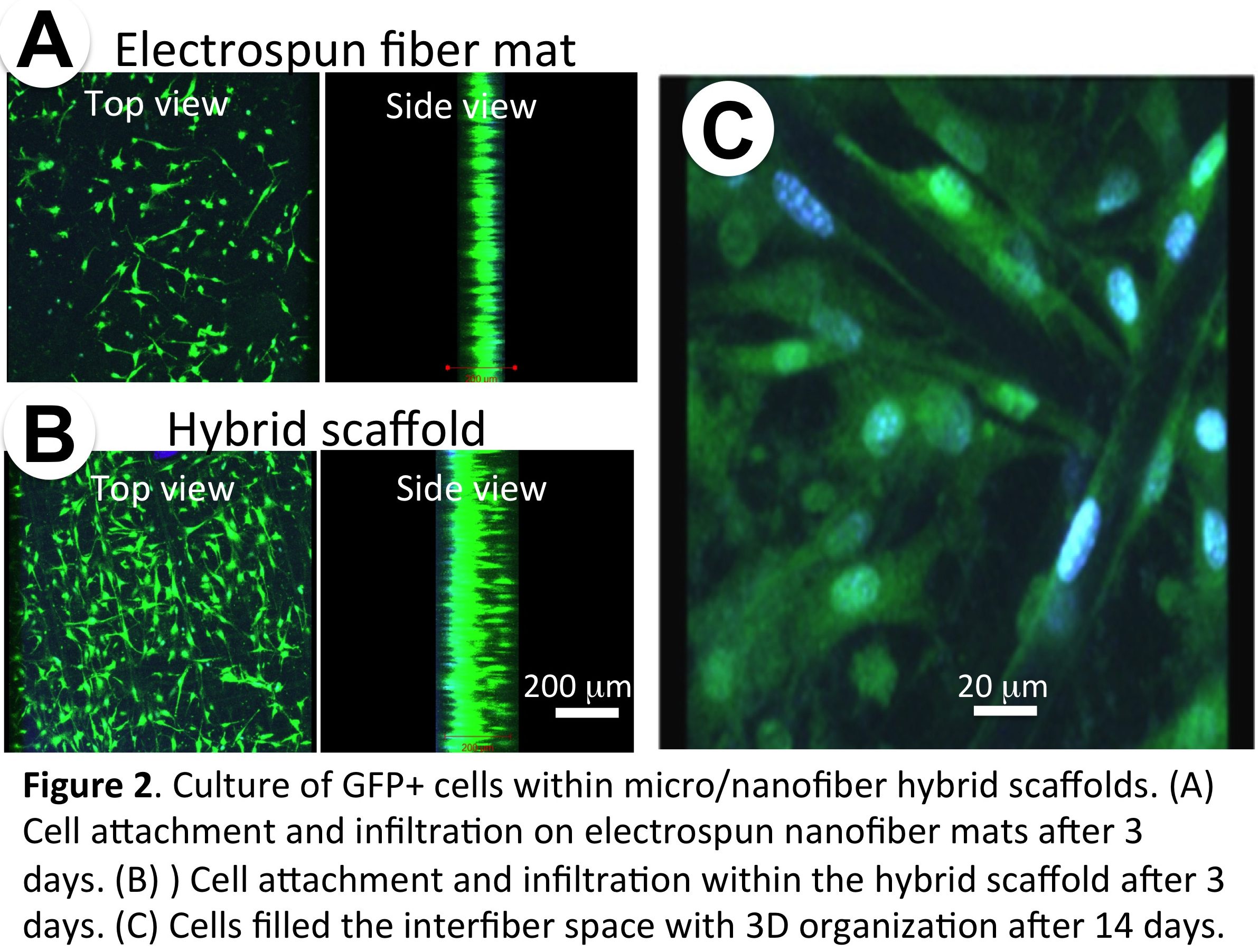
Cell culture within micro/nanofiber scaffolds: Mouse preosteoblasts (MC3T3) were seeded and cultured on the hybrid scaffolds or electrospun nanofiber mats. Cell distribution and tissue formation within the scaffolds were characterized by fluorescence microscopy, confocal microscopy and histological analysis.
Results and Discussion: With the optimized conditions, micro/nanofiber hybrid scaffolds were successfully fabricated (Figure 1A&B), in which the PCL microfibers provide large pores for cells to infiltrate and good mechanical strength (Figure 1C), while the PCL/collagen nanofibers support cell adhesion and proliferation. Different from PCL/collagen electrospun mats, in which cells dominantly attached to the mat surface due to the small pore size (< 5mm), the hybrid scaffolds allowed cell infiltration throughout the entire thickness with high uniformity and the cells exhibited three-dimensional organization within the microfiber grids (Figure 2). Upon prolonged culture, homogeneous tissue formation within the hybrid scaffolds was observed with the deposition of minerals and expression of alkaline phosphatase.
Conclusion: Clearly, the hybrid scaffolds have multifaceted advantages, in terms of the increased cell infiltration, well-controlled structure and improved mechanical performance, compared to electrospun fiber mats. Meanwhile, the culture of osteoblasts within such scaffolds leads to the formation of bone-like tissue with uniform cell distribution and new ECM formation.
This study was financially supported by the Biomaterials program of the National Science Foundation (DMR 1508511)
References:
[1] C. Erisken, D.M. Kalyon, H. Wang. Functionally graded electrospun polycaprolactone and β-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials 2008, 29, 4065.
[2] X. Yang, K.R. Ogbolu, H Wang. Multifunctional nanofibrous scaffold for tissue engineering. Journal of Experimental Nanoscience 2008,3,329.