Introduction: Injectable hydrogels with shear-shinning properties have emerged as promising biomaterials due to their permeability, biocompatibility, and ease-of-use through minimally-invasive procedures. Strain-responsive hydrogels provide a convenient and effective approach to administer a wide variety of bioactive agents such as genes, proteins, drugs, and living cells. The goal of this study was to develop a dual- responsive, injectable alginate hydrogel based on the supramolecular complex formation between β-cyclodextrin (β-CD, grafted onto the alginate repeat unit) and the poly(propylene glycol) (PPG) component of Pluronic® F108. The conjugation of cysteine-arginine-glycine-aspartic acid-serine (CRGDS) onto the alginate backbone was investigated to construct a 3-D, cell-instructive hydrogel.
Materials and Methods: ρ-toluenesulfonyl (TosCl) chloride, dissolved in acentontrile, was added to aqueous β-CD solution at 4˚C to synthesize β-CD-TosCl. A 3:1 equivalent of NaOH was added drop-wise and stirred for 30 min at RT. The product was washed with ice water and acetone and dried under vacuum[1]. β-CD-TosCl was mixed with ethylenediamine (EDA) under N2 at 60˚C and reacted for 12 h. Alg-g-CD-CRGDS was synthesized from alginate and β-CD-EDA via carbodiimide chemistry[2]. Hydrogels were prepared from Alg-g-CD solutions mixed with Pluronic® F108 powder[3]. Shear-thinning experiments were performed on an AR2000 (TA Instruments) to test the reversibility of the hydrogel structure and thus mechanical properties; experiments started with low radial strain (0.5%) and then increased to a high radial strain (250%); each cycle was repeated 2x at 37 °C, 1 Hz. Storage (G’) and loss (G”) moduli were recorded during a temperature sweep at a heating rate of 0.5°C/min at 1 Hz frequency. Human lung cancer cells (A549 ATCC® CCL-185™) were mixed with 4% (w/v) Alg-g-CD-CRGDS and Pluronic® F108 (1:8 molar ratio) solutions, and ejected from an 18G needle at a concentration of 1 million cells/mL. The 3-D cell constructs were cultured at 37˚C, 5% CO2, stained with Live/Dead® overnight, and imaged using confocal laser scanning microscopy.
Results: Figure 1 illustrates in situ hydrogel formation between Alg-g-CD-CRGDS and Pluronic® F108. The formation of the supramolecular inclusion complex was confirmed by rheometry.
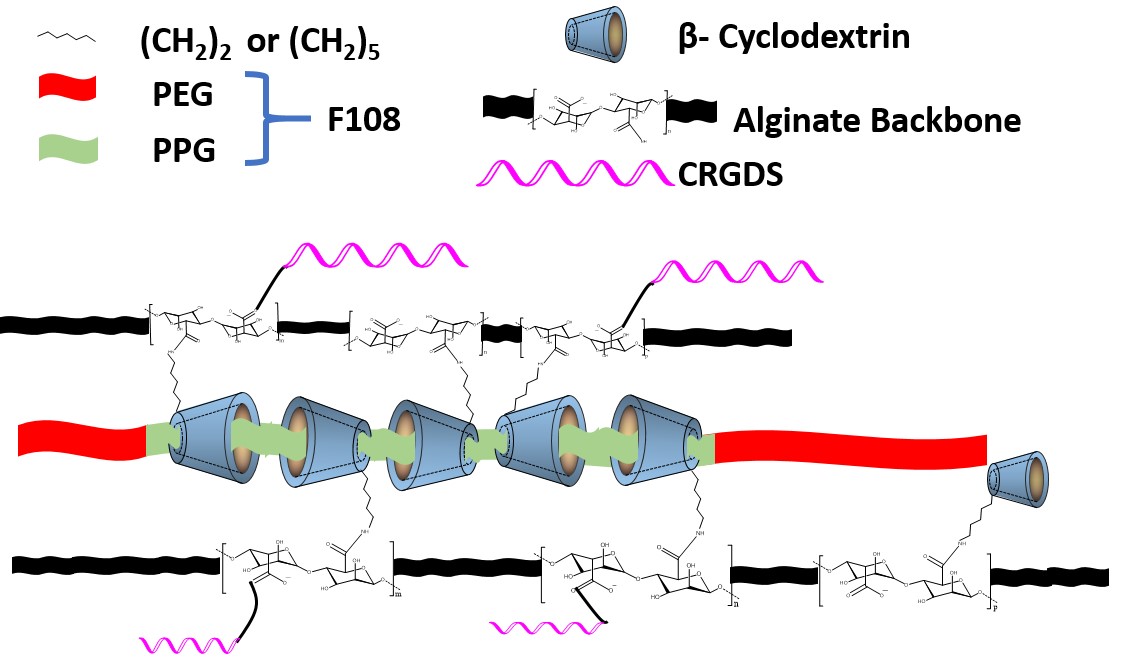
Figure 2A showed the chemistry synthesis of Alg-g-CD-CRGDS Figure 2B shows stacked 1H-NMR spectra for alginate (red), Alg-g-CD (green) and Alg-g-CD-CRGDS (blue), indicating the successful conjugation of β-CD and CRGDS onto the alginate backbone.
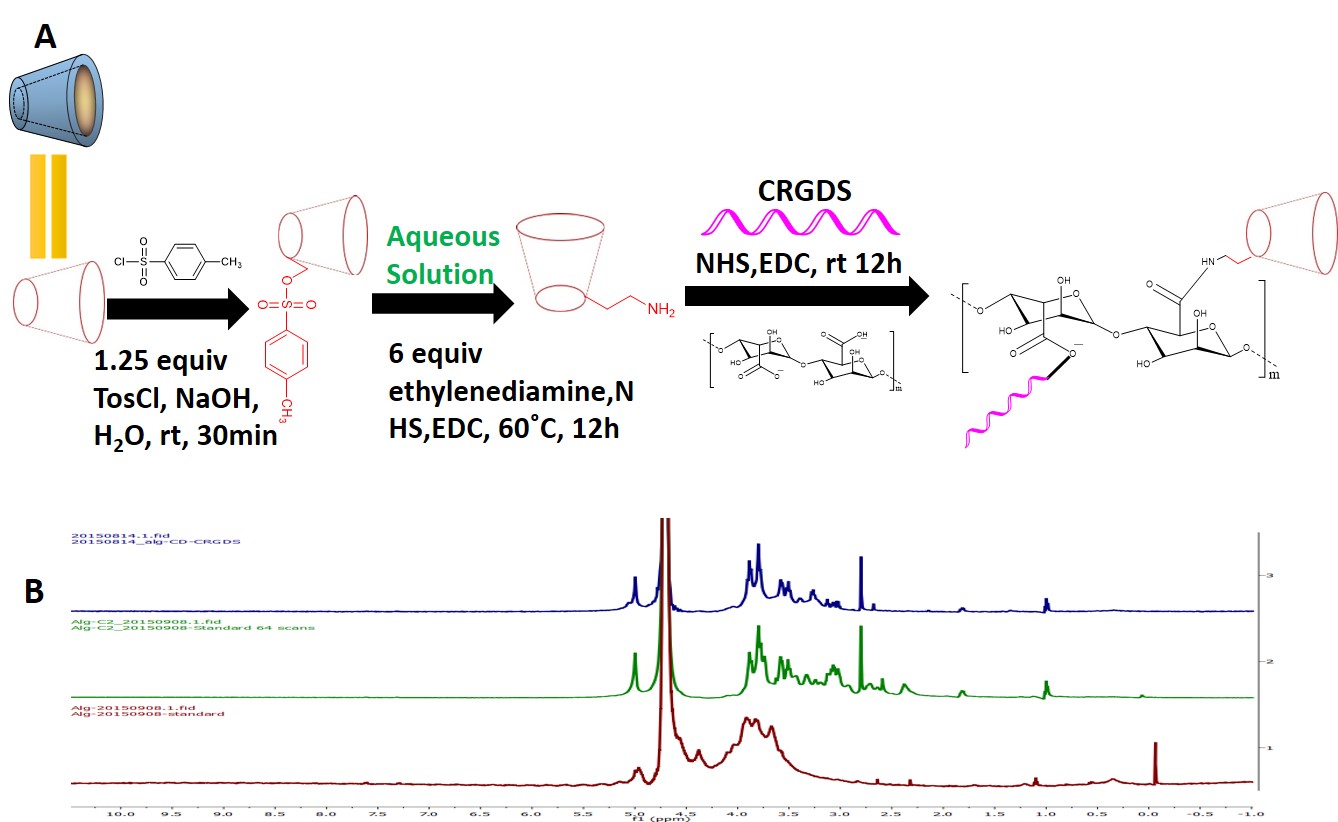
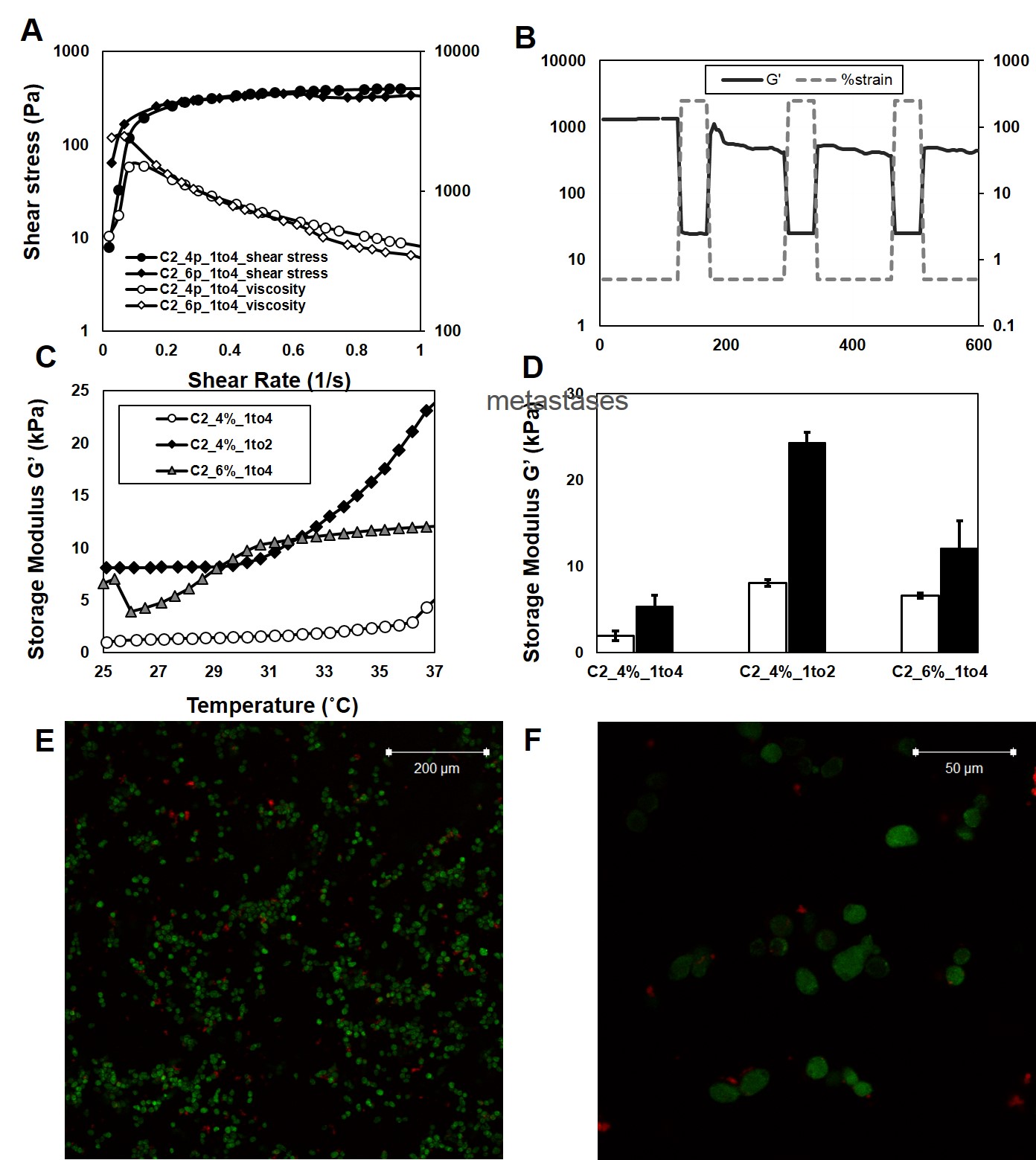
Figure 3A demonstrates the dynamic relationship between shear stress and shear rate for Alg-C. Figure 3B demonstrates a self-curing and repeatable ability to deform and re-assemble upon loading and un-loading. Figure 3C illustrates the thermo-response of Alg-g-CD-CRGDS:F108 hydrogels and Figure 3D displays shear storage moduli (G’) for Alg-CD:F108 hydrogels at 25°C (white bars) and 37°C (black bars). A significant difference in G’ was observed between 25 and 37˚C within the same sample group. Figure 3E/3F demonstrates A549 cell distribution in the 3-D injectable hydrogel. Cells were alive and homogenously distributed throughout the hydrogel structure.
Conclusion: The multi-stimuli responsive alginate-based hydrogels, with covalently attached cell instructive peptides (i.e., CRGDS) and tunable mechanical properties, warrant further investigation for biomedical applications such as drug delivery and cell transplantation in vivo.
The authors would love to thank Dr. Monka. Ivancic for the help in 1H-NMR spectra.
References:
[1] Rodell, C. B.; Kaminski, A. L.; Burdick, J. A., Rational Design of Network Properties in Guest–Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules 2013, 14, (11), 4125-4134.
[2] Izawa, H.; Kawakami, K.; Sumita, M.; Tateyama, Y.; Hill, J. P.; Ariga, K., [small beta]-Cyclodextrin-crosslinked alginate gel for patient-controlled drug delivery systems: regulation of host-guest interactions with mechanical stimuli. Journal of Materials Chemistry B 2013, 1, (16), 2155-2161.
[3] Lin, N.; Dufresne, A., Supramolecular hydrogels from in situ host-guest inclusion between chemically modified cellulose nanocrystals and cyclodextrin. Biomacromolecules 2013, 14, (3), 871-80.