Introduction: Understanding the influence of the chemical environment within the extracellular matrix (ECM) on cellular activities is crucial to the fields of cancer and stem cell biology. ECM mimics are necessary to elucidate the role of cell-ECM interactions, which will lead to new biomaterials for regenerative therapies. The chemical composition of the ECM is spatially defined and dynamic, which are important regulators of cell fate[1]-[3]. In an effort to understand the dynamic features of the ECM that control cellular processes, we have developed ECM mimics from hydrogels where the biochemical environment can be temporally controlled. We are now expanding the system for the simultaneous spatial and temporal patterning of biomolecules within hydrogels using photochemistry.
To achieve temporal control of the biochemical environment, peptides were immobilized in hydrogels through high affinity reversible physical interactions. Peptide-streptavidin conjugates were immobilized in agarose-desthiobiotin (AgD) hydrogels through the desthiobiotin-streptavidin interaction (KD 10-11M), and displaced by adding biotin, which binds streptavidin with greater affinity than desthiobiotin (KD 10-15M)[4]. This cycle was repeated to vary the chemical environment over time (Fig. 1). Photocaged peptides, which become bioactive upon irradiation, are now being immobilized using the desthiobiotin-streptavidin system to yield spatial control.

Material and Methods: Synthesis of materials. AgD was synthesized by reacting amino-agarose with NHS-desthiobiotin. Peptide-streptavidin conjugates were synthesized by reacting maleimide-streptavidin with cysteine containing peptides such as CGRGDS. Peptide-streptavidin conjugates were fluorescently labelled with NHS-Alexa Fluor dyes.
Reversible biomolecule immobilization. Immobilization experiments were performed by adding solutions of fluorescent streptavidin (with or without conjugated RGD) to 0.7 wt.% AgD gels. Biotin was added to unbind streptavidin. After a washing step to remove free biotin, streptavidin conjugates were re-immobilized within the same gels. Experiment were performed in PBS or media with 0.5 % BSA or 1 % calf bovine serum.
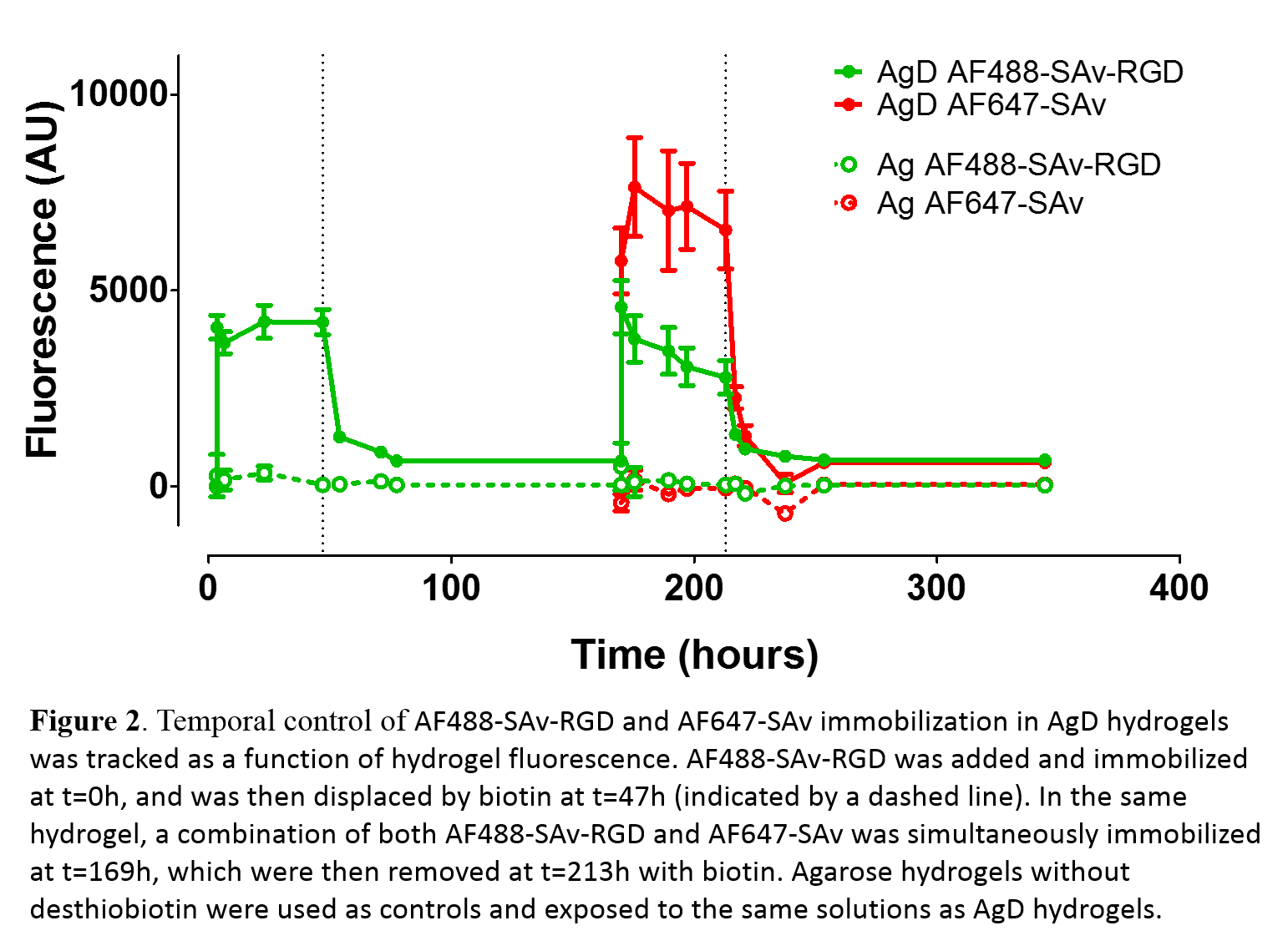
Results and Discussion: Sequential immobilization of the adhesive RGD peptide in AgD hydrogels was achieved by varying media components. To demonstrate temporal control, we bound and unbound 2 different fluorescent molecules: 1) streptavidin modified with CGRGDS and Alexa Fluor 488 (AF488-SAv-RGD); and, 2) streptavidin modified with Alexa Fluor 647 (AF647-SAv) as a surrogate for a second biomolecule (Fig. 2). AF488-SAv-RGD was added at t=0h, which resulted in biochemically stable hydrogel (stable for over 20days). Biotin was introduced at t=47h (dashed line in Fig. 2) to remove AF488-SAv-RGD, as seen from the loss of hydrogel fluorescence. Simultaneous immobilization of 2 different molecules was achieved by adding a solution of AF488-SAv-RGD and AF647-SAv at t=170h. Unbinding was again achieved at 213h through the addition of biotin. Control experiments were conducted with agarose (Ag) hydrogels to quantify non-specific binding. This data demonstrates that it is possible to temporally control the biochemical environment of hydrogels using strong reversible physical interactions. It was also demonstrated that streptavidin-RGD is bioactive by performing fibroblast adhesions assays.
Conclusion: We have developed a system to easily tune the biochemical environment of ECM mimics over time. We are currently expanding the system to control the biochemical environment in space and time through the use of photocaged peptides. This system will yield tailored biochemical environment to study and control cell-matrix interactions for applications in regenerative medicine. Furthermore, the method will be useful for many studies involving cell-matrix interactions due to its simplicity.
This work was supported by NSERC USRA (C.L.), NSERC Discovery Grant and McMaster University.
References:
[1] Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. The Journal of cell biology. 2012;196:395-406.
[2] Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nature materials. 2014;13:547-57.
[3] Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433-41.
[4] Hirsch JD, Eslamizar L, Filanoski BJ, Malekzadeh N, Haugland RP, Beechem JM, et al. Easily reversible desthiobiotin binding to streptavidin, avidin, and other biotin-binding proteins: uses for protein labeling, detection, and isolation. Analytical biochemistry. 2002;308:343-57.