Introduction: Layered microparticles (MPs) formulated using poly(L-lactic acid) (PLA) and poly(D,L-lactic-co-glycolic acid) (PLGA) have been widely investigated for delayed release of small molecule drugs. However, the extension to protein delivery is limited even though temporal presentation/activity of proteins is critical for cellular signalling. Additionally, most reports rely on complex tools (i.e. tricapillary electrosprayer) to successfully fabricate layered MPs[1]. Here, we report a straightforward protocol for: 1) encapsulating the model protein, bovine serum albumin (BSA) in core-shell MPs, and 2) modulating the protein release profile using ethanol (EtOH).
Materials and Methods: Protein-loaded PLGA-PLA particles were formulated by adapting a previously described technique to encapsulate BSA[2]. Both PLGA and PLA were dissolved in dichloromethane where EtOH was added (0, 2.5 & 5%; v/v) to the PLA solution. The aqueous protein phase was mixed with the PLGA solution, transferred to the PLA solution and emulsified in a 4x volume excess of 1.0% (w/v) poly-vinyl alcohol (PVA) using a table-top vortexer. After solvent evaporation in 15x excess of 0.5% PVA, the MPs were recovered by subsequent washes and lyophilization. Particle size and architecture were evaluated through scanning electron microscopy (SEM). Release profiles were determined from protein loading and release assays. Protein localization (fluorophore-conjugated insulin) within particles was quantified using confocal microscopy and MATLAB.
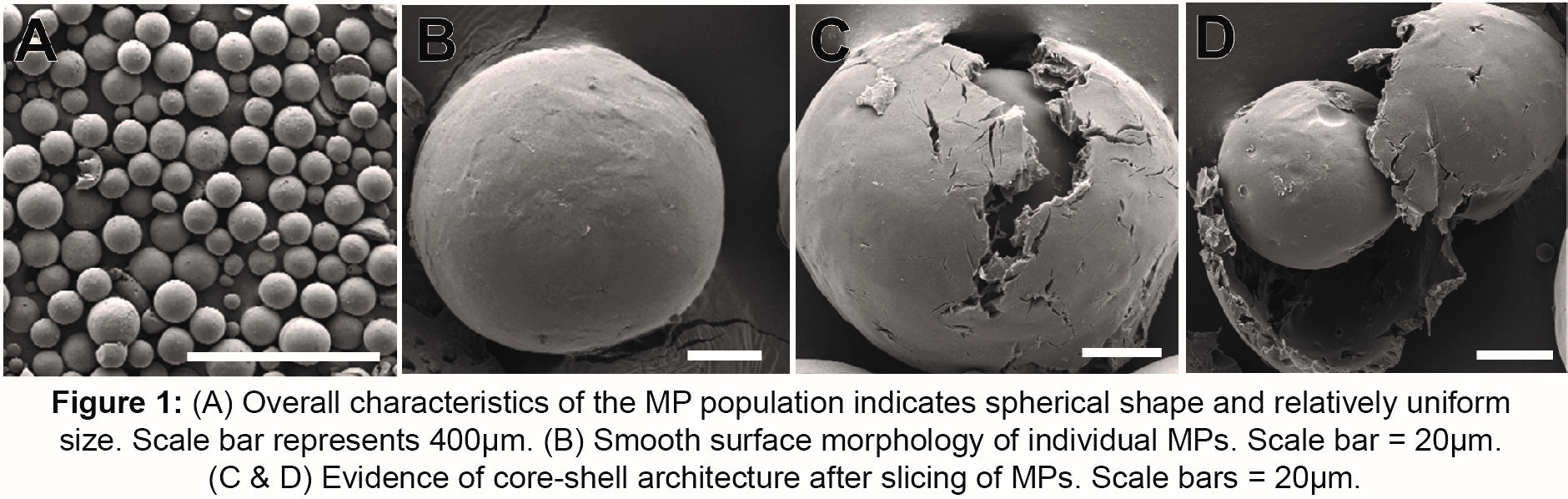
Results: Average particle diameters ranged between 32-44µm and showed an increasing trend with the addition of EtOH. SEM images of freshly prepared and DMSO exposed particles verified a core-shell architecture with a PLGA-rich core and a PLA-rich shell (Figures 1 & 2). Protein encapsulation efficiencies were between 65-73% and release profiles indicate an initial burst release between 12-20% of cumulative cargo. Marked differences are seen in cumulative release over 40days: 32%, 43%, and 100% for 0%, 2.5% & 5% (v/v) EtOH groups, respectively (Figure 3A). Confocal images indicate protein localization within the core of PLGA-PLA MPs. Upon addition of EtOH, protein distribution was more diffuse across MPs (Figure 3B).

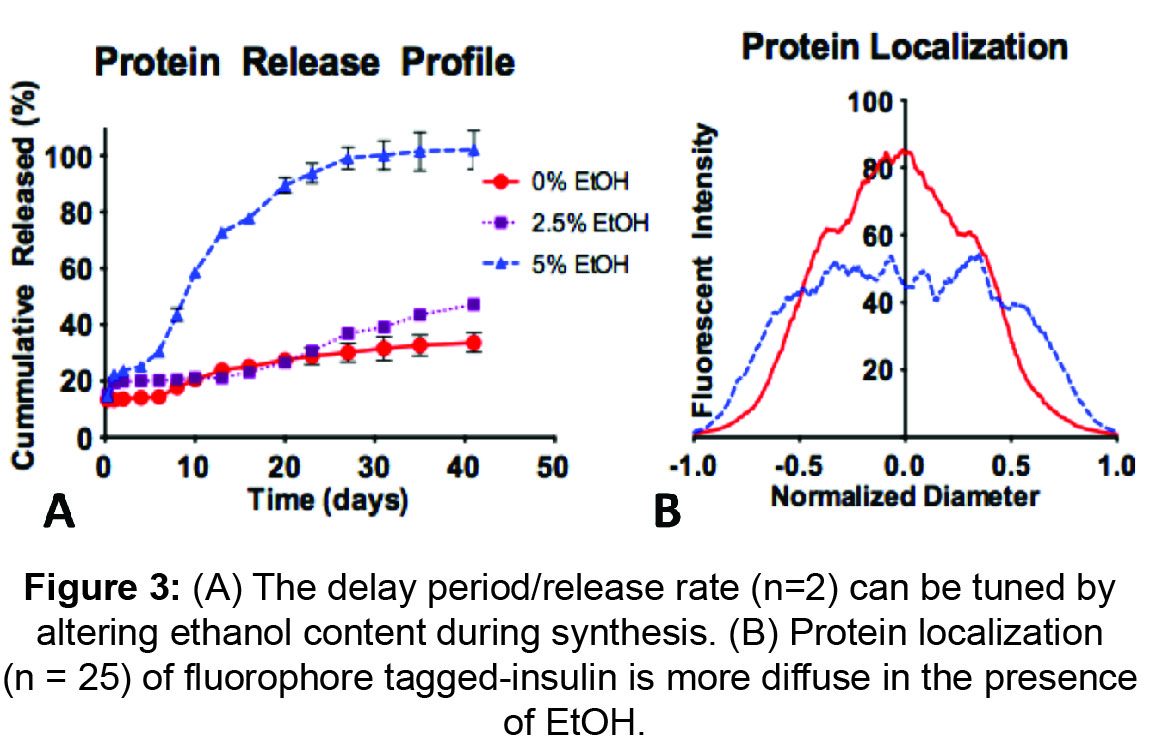
Discussion: To our knowledge, we are the first to demonstrate that adding EtOH in the shell phase modulates release profiles in core-shell (PLGA-PLA) MPs. EtOH likely influences: 1) protein mobility within the oil phase, and 2) infiltration of aqueous continuous phase into the dispersed oil phase during solvent evaporation. The result is a more diffuse protein distribution (Figure 3B) within MPs and a relatively porous surface morphology (as seen from SEM). Release profiles indicate EtOH can modulate protein release rate and delay period.
Conclusion: BSA was encapsulated in core-shell PLGA-PLA particles using a straightforward protocol and we report ethanol as a novel means for tuning their release profile.
Dr. Rachael Sirianni, Barrow Neurological Institute, Phoenix, AZ; John M. Cowley Center for High Resolution Electron Microscopy, Tempe, AZ
References:
[1] Y. Xia, Q. Xu, C. Wang and D. W. Pack, J. Pharm. Sci., 2013, 102, 1601–1609.
[2] T. H. Lee, J. Wang and C.-H. Wang, J. Controlled Release, 2002, 83, 437–452