Recapitulating vascular interfaces of different organs in 3-D is critical in both organ-on-a-chip[1] and tissue engineering applications[2]. 3-D micro-tissues composed of parenchymal cells have often been studied in the absence of vasculature, whereas vasculature-on-a-chip has primarily been studied separately from parenchymal cells. Furthermore, clinical translation has been achieved only for thin tissues or those with a low metabolic demand (e.g. skin, cartilage and bladder). Large solid tissues (e.g. myocardium, liver) are highly sensitive to oxygen levels and become vulnerable within hours without oxygen supply. These tissues would greatly benefit from rapid vascularization in vitro and direct vascular integration in vivo.
Vascular networks can be engineered with subtractive fabrication by embedding a sacrificial carbohydrate-glass lattice[3], Pluronic F127[4], or dry alginate fibers[5] in hydrogels. However, the soft hydrogel provides only a temporary structural support for the fragile hollow network and does not permit extensive tissue remodeling[3], which inevitably alters the hydrogel structure and collapses the embedded network. Synthetic biodegradable polymers could provide sufficient structural support to the engineered vessels, but their low permeability prevents biomolecule exchange and cell migration between the vessels and the parenchymal space.
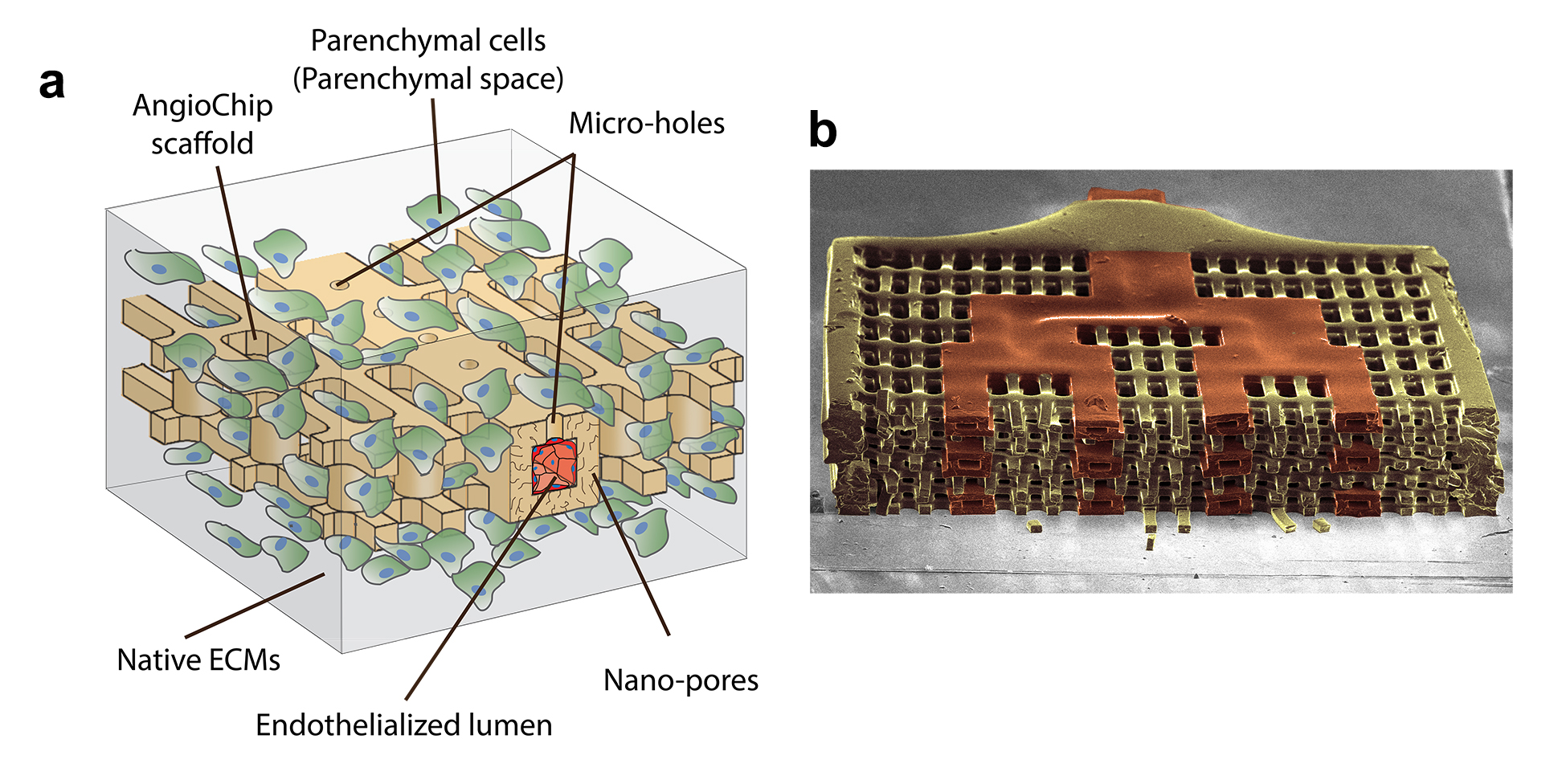
To accommodate these two opposing material criteria we created AngioChip, a stable biodegradable scaffold with a built-in branching micro-channel network (Figure 1) that contained two unique features realized by our new 3-D stamping technique. First, the synthetic built-in vascular walls were thin and flexible, yet strong enough to mechanically support a perfusable vasculature in a contracting tissue and enable direct surgical anastomosis. Second, to allow efficient molecular exchange and cell migration, nano-pores and micro-holes were incorporated into the vascular walls. By establishing a stable, permeable, vessel network within AngioChips, we were liberated from material constraints, which allowed us to use any soft natural extracellular matrix (e.g. collagen, Matrigel) embedded with cells in the parenchymal space permitting the extensive tissue remodelling.
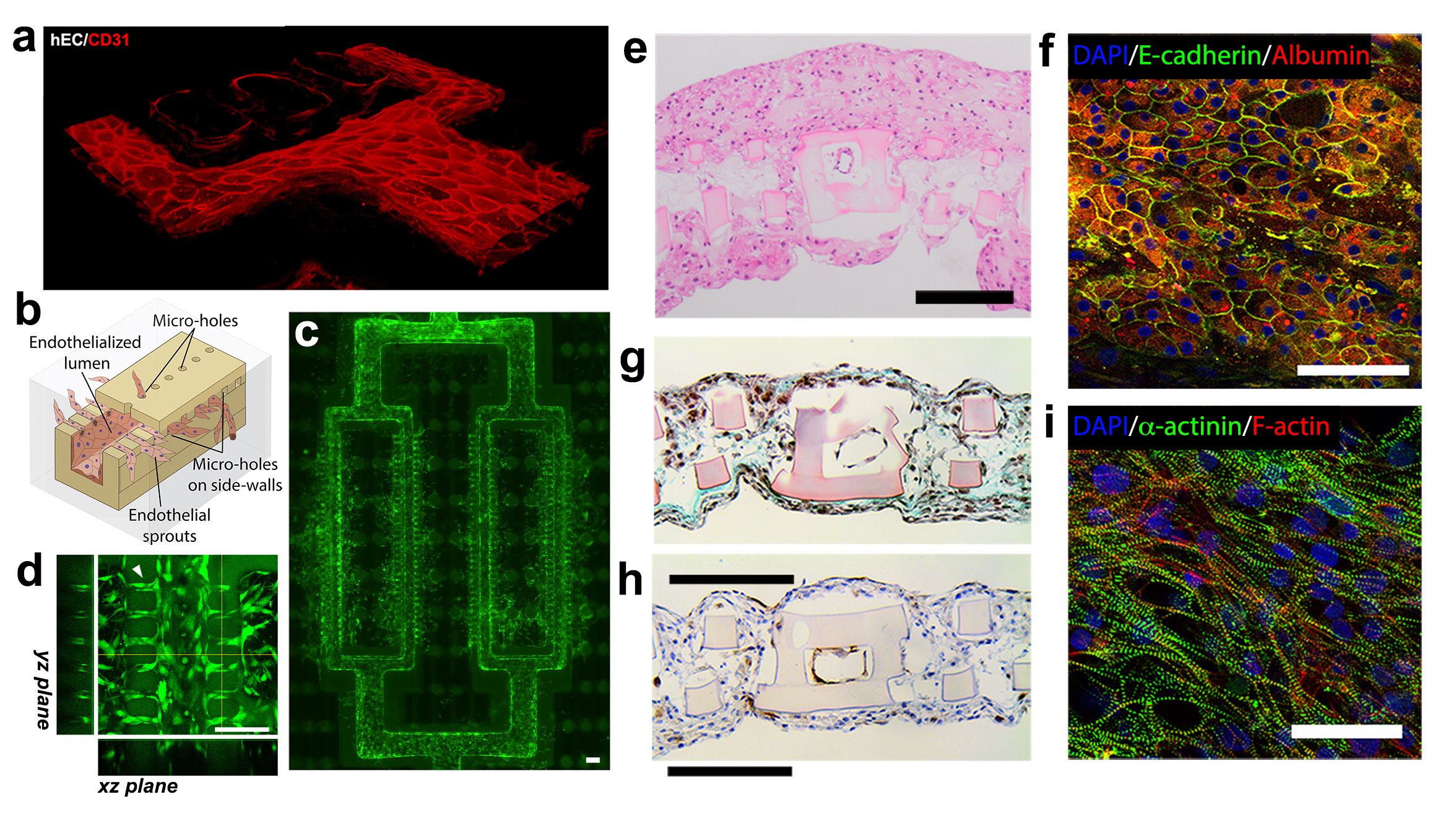
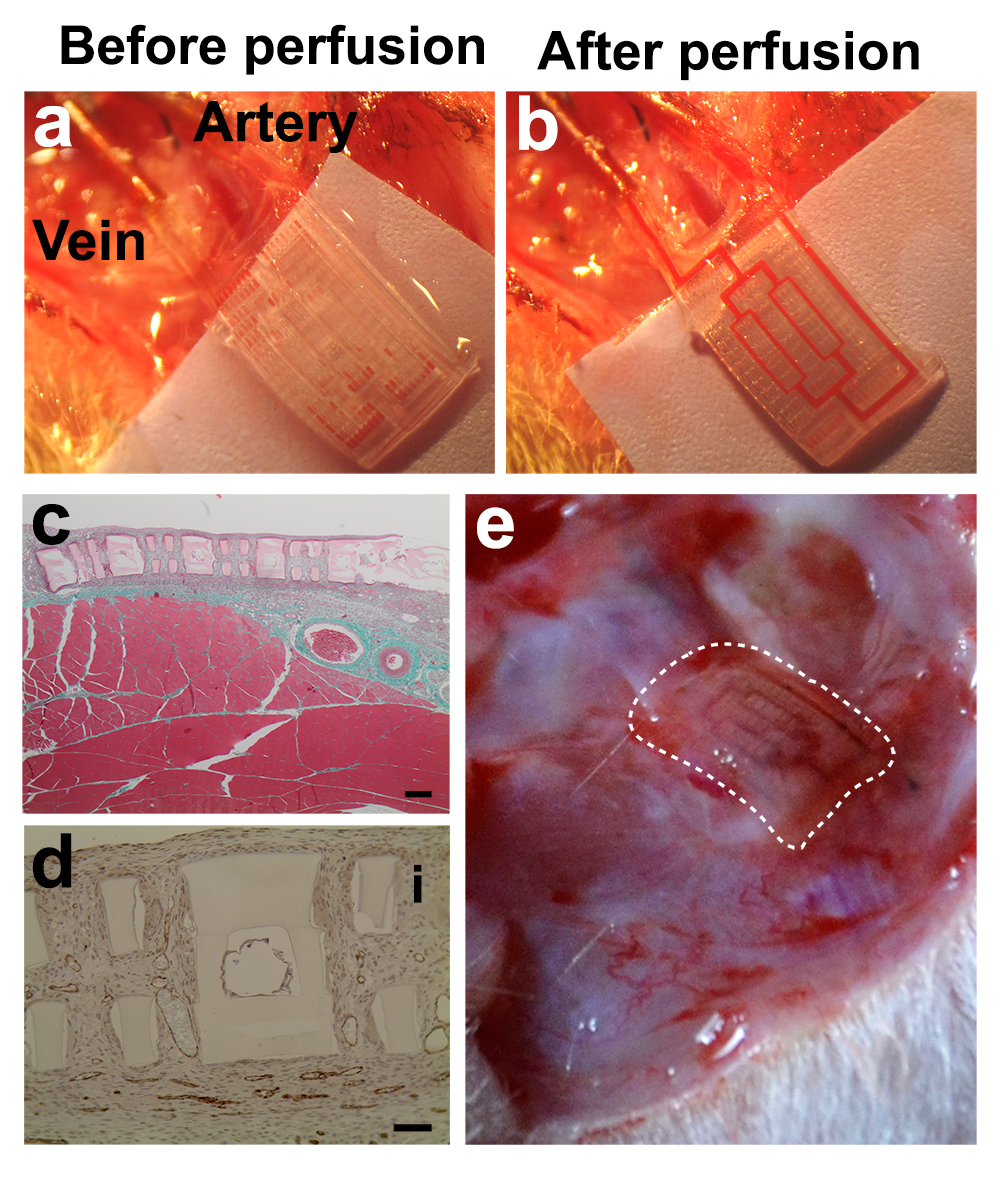
Cardiac tissues were created from human embryonic stem cell (hESC), human mesenchymal stem cells (MSCs) as a side population and HUVECs for inner lumen coating. To provide an evidence of tissue-level organization, confocal images and histological cross-sections show compact layers of cells throughout the entire tissue volume, including the scaffold interior (Figure 2g,h,i). Fully human liver-AngioChips were engineered using hESC derived hepatocytes. High-density culture resulted in the formation of junctions between hepatocytes, and a positive staining for albumin and bile canaliculi (Figure 2e,f). Vascularized hepatic tissues and cardiac tissues, engineered using AngioChips, were shown to process clinically relevant drugs delivered through their internal vasculature. Incorporation of nano-pores and micro-holes in the vessel walls enhanced vessel permeability, permitted inter-cellular crosstalk, extravasation of monocytes and sprouting of endothelial cells upon stimulation (Figure 2a,b,c,d). AngioChip cardiac tissues were also implanted via direct surgical anastomoses to the femoral vessels of rat hindlimbs, establishing immediate blood perfusion (Figure 3).