Introduction: Myocardial infarction kills one in six people in the US, due to an inability to regenerate cardiac muscle lost after infarct. Cardiac muscle engineered in vitro is a potential approach to create new myocardium for infarct repair, however multiple challenges exist. Specifically, while spatially well-defined micropatterns of ECM proteins such as fibronectin (FN) have been shown to direct cardiomyocyte (CM) organization on 2D surfaces, extending this micropatterning to 3D scaffolds has been difficult. To address this, we have developed a technique to micropattern directional FN cues into 3D hydrogel scaffolds to direct CM organization. We compared CM alignment in response to FN lines on collagen type I (COL1) or fibrin hydrogels, with or without Matrigel and identified a specific scaffold composition that maximized CM uniaxial alignment. We then used this technique to build aligned 2D human CM sheets and multilayered 3D tissues.
Methods: To engineer micropatterned hydrogel scaffolds, we modified a previously reported surface-initiated assembly technique[1]. Briefly, 20 μm wide FN lines were microcontact printed onto a sacrificial poly(N-isopropylacrylamide) (PIPAAm) substrate. PIPAAm was then dissolved to transfer the FN lines, first on gelatin, then onto hydrogels consisting of COL1 or fibrin with or without Matrigel. Embryonic chick CMs were cultured for 4 days onto patterned hydrogels, then fixed and stained for nuclei, actin, and α-actinin, with CMs seeded on micropatterned polydimethylsiloxane used as controls. Similarly, human embryonic stem cell derived CMs were seeded on COL1 patterned with FN lines. Orientational order parameter (OOP) of the actin cytoskeleton was analyzed to determine the hydrogel composition that maximized alignment of both CM types. To create multilayered constructs, engineered cardiac tissues were cultured for 2 days, stacked, and maintained in contact for 3 days before fixing.
Results: FN patterns were well defined on the COL1 and fibrin, with <10% variation in line width. Chick CM alignment on fibrin was low for all concentrations of fibrin and Matrigel (OOP<0.5). In contrast, CMs aligned well on COL1 without Matrigel (OOP = 0.66 vs. 0.38 with Matrigel), and reached maximum anisotropy on high concentration COL1 (6 mg/mL) without Matrigel (OOP=0.75±0.1). Human CMs seeded on the high COL1 concentration substrate formed anisotropic contractile monolayers with distinct sarcomeres (OOP = 0.65±0.1). To build 3D tissues, we stacked cardiac sheets 3 layer high with a single layer thickness of 17 ± 1.8 µm.
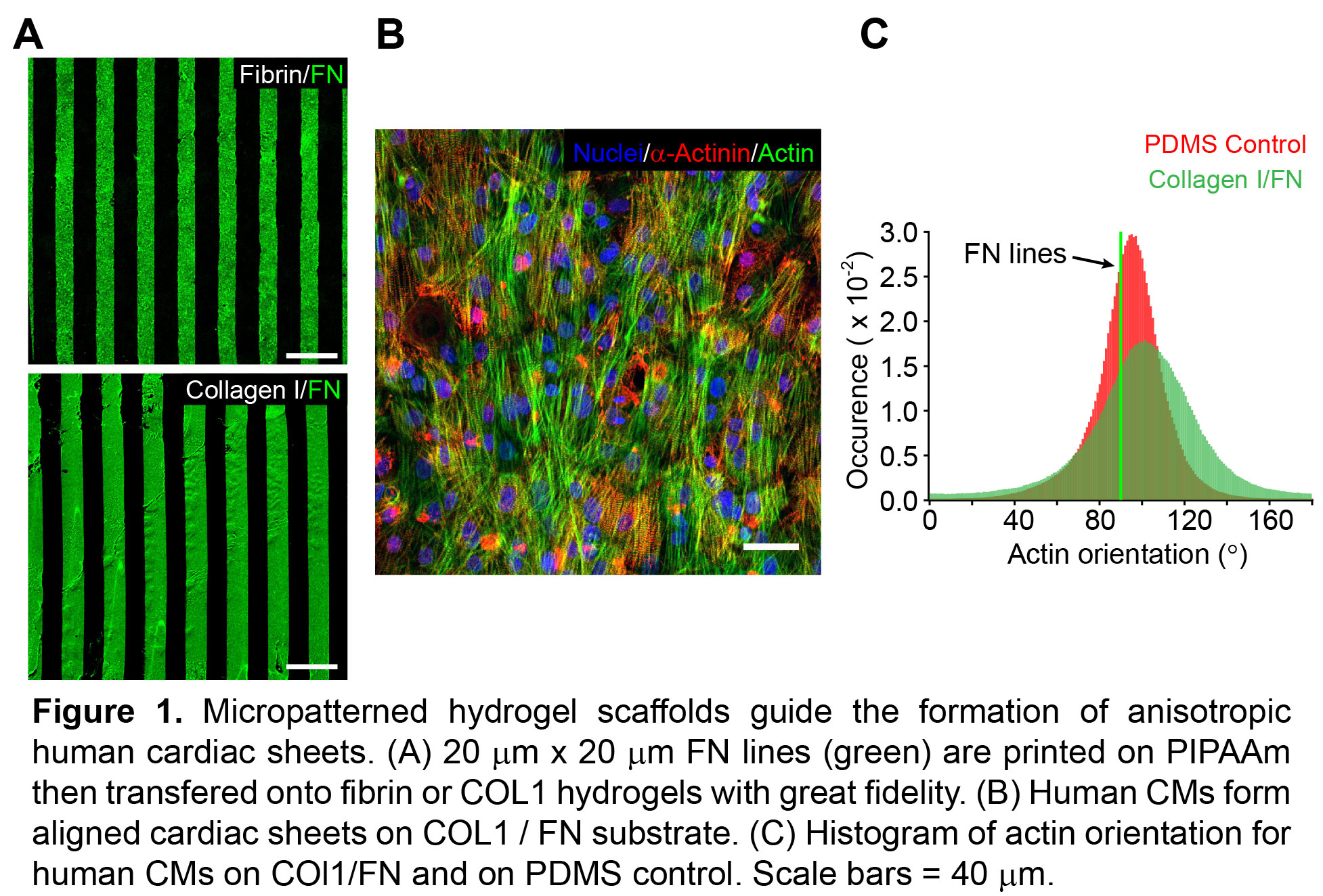
Conclusions: We engineered directional, microscale ECM cues into hydrogel scaffolds and successfully guided the alignment of cardiac cell sheets, including human CMs. The high concentration COL1 with integrated FN micropatterns maximized both chick and human CM alignment. Future work will focus on engineering multi-layered and aligned human cardiac tissues and assessing electrophysiology and contractility.
Financial support from the Dowd-ICES fellowship to Q. Jallerat and from National Institutes of Health 1DP2HL117750 and American Heart Association 12SDG11800036 to A. W. Feinberg.
References:
[1] A. W. Feinberg, et al, Nano Lett. 2010, 10, 2184