Introduction: Here we report on the development of a new approach for low-dose prostate cancer brachytherapy, based on the intra-tumoral injection of radioactive nanoparticles (103Pd:Pd@Au-PEG NPs). The radioisotope palladium (103Pd; 20 keV photons; 17-days half-life), encapsulated in millimetre-size seed implants, is widely used in prostate cancer brachytherapy (internal radiotherapy). It was reported that the interaction of low-energy 103Pd photons with gold (Au), produce a large number of photoelectric events that are expected to lead to higher energy deposition and strongly enhance the dose to tumors[1]. Therefore, injections of 103Pd-doped Au NPs could represent an alternative to implants, which are associated to discomfort and dose heterogeneities.
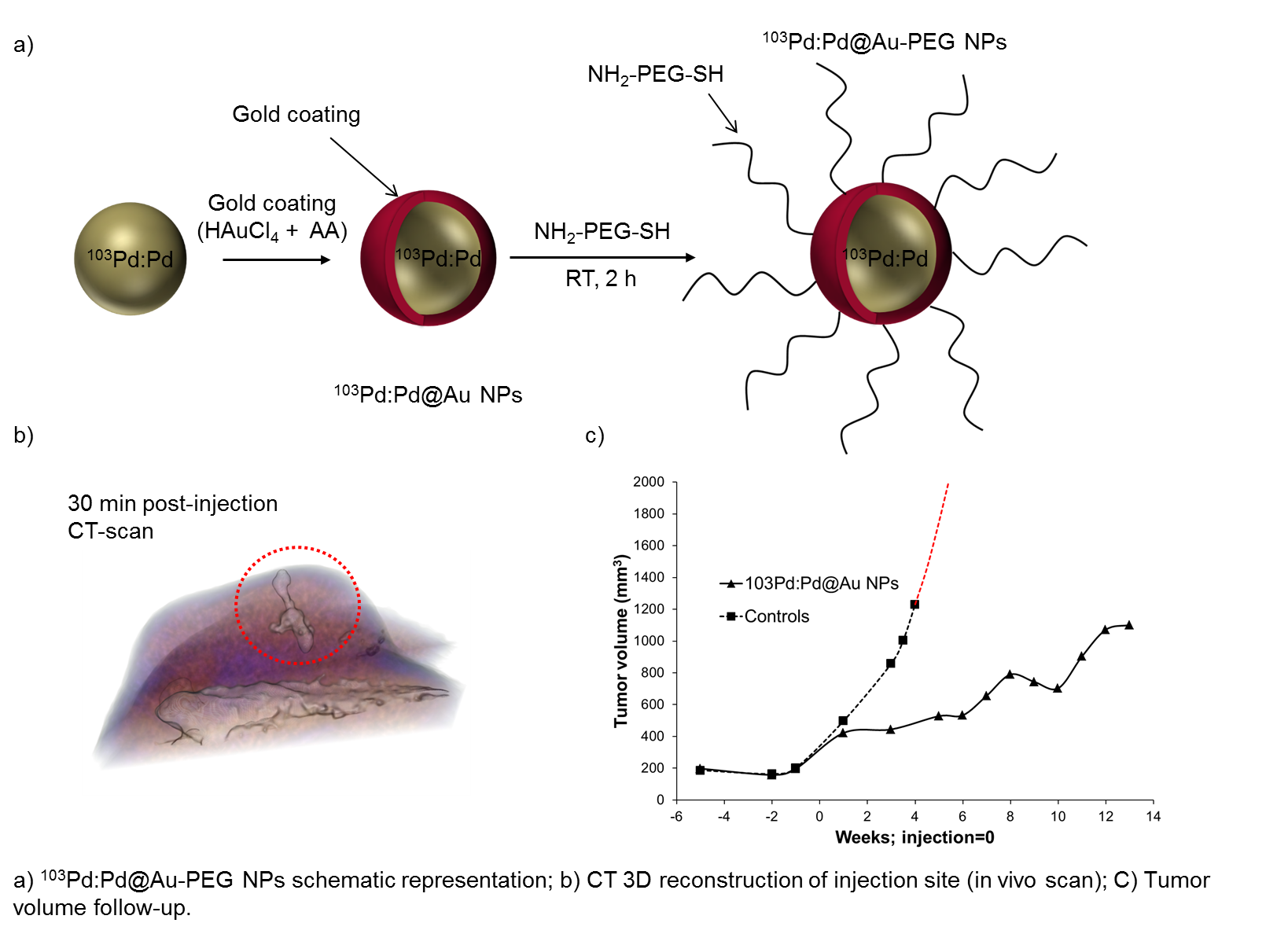
Materials and Methods: First, a rapid one-pot procedure was developed to synthesize ultra-small 103Pd:Pd nanoparticles (radioactive precursor: 103PdCl2, MDS Nordion), followed by encapsulation with gold. Thus-obtained core-shell nanoparticles (5-15 nm dia.) were stabilised with biocompatible polyethylene glycol (NH2-PEG-SH), purified and concentrated by centrifugation[2]. Then, aqueous alginate solution (2% w/v) was added to the NPs suspension. Alginate molecules polymerize in contact of Ca2+ ions present in the body, and form a crosslinked gel, allowing the sequestration of the NPs at the tumor site at the time of injection[3]. The therapeutic effect of 103Pd:Pd@Au-PEG NPs was studied in a PC3 prostate cancer xenograft model (n = 10). Nanoparticles (4 μL; 1.7 mCi) were injected over 2 min with a precision micropump. CT-scans were performed immediately after injection and one week later. Tumor volume follow-up was measured with MRI (over a period of 14 weeks) and regions of interest (ROIs) were drawn over tumor section. Finally, an organ biodistribution study was also performed. The organs (tumor, liver, spleen, kidneys, lungs, heart) were harvested and radioactivity-counted.
Results and Discussion: The 103Pd:Pd@Au-PEG NPs injection sites were clearly identified and delineated on CT images. Volume reconstruction was performed on each injection site, in order to quantify NPs diffusion (at t = 30 min and t = 1 week). These observations confirmed that alginate gel is entrapping the NPs at the tumor site. Tumor follow-ups showed a significant inhibition of tumor growth after a single dose administration. Tumor volumes growth of treated animals appeared to be delayed in comparison to control group. In 9/10 animals, the total activity retention in the tumors was higher than 75% of the total activity in the animal. However, 15 % was found in the liver, indicating that a fraction of NPs is taken up by the tumor vasculature and immune cell processes, ending up in the organs associated to the reticuloendothelial system (liver, spleen).
Conclusion: Small radioactive nanoparticles (103Pd:Pd@Au-PEG) have been efficiently synthesized and successfully injected in prostate cancer xenograft tumors. Moreover, the crosslinked alginate gel allowed a good retention of the NPs in tumors. Also, therapeutic effect of radioactive nanoparticles was clearly demonstrated by the inhibition of tumor growth. Overall, this study confirms the potential of 103Pd:Pd@Au-PEG nanoparticles for more precise and less invasive brachytherapy procedures.
References:
[1] Lechtman E, Chattopadhyay N, Cai Z, Mashouf S, Reilly R, Pignol JP. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Physics in Medicine and Biology 2011 Aug 7;56(15):4631-4647.
[2] Djoumessi D, Laprise-Pelletier M, Chevallier P, Lagueux J, Cote MF, Fortin MA. Rapid, one-pot procedure to synthesise 103Pd:Pd@Au nanoparticles en route for radiosensitisation and radiotherapeutic applications. Journal of Materials Chemistry B 2015;3(10):2192-2205.
[3] Le Renard P-E, Jordan O, Faes A, Petri-Fink A, Hofmann H, Rüfenacht D, Bosman F, Buchegger F, Doelker E. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials 2010;31(4):691-705