The Adjuvant Effect Of PLGA Is Mediated Via Dendritic Cells In Vivo
-
1
Georgia Institute of Technology, Wallace H. Coulter Department of Biomedical Engineering, United States
Introduction:
The number of applications using biomaterials is rapidly growing due to the diversity of physical and chemical properties they can offer; specifically, devices comprised of materials such as poly lactic-co-glycolic acid (PLGA) have been approved by the Food and Drug Administration (FDA) for clinical use. We have previously shown that PLGA is distinct in its interaction with the adaptive immune system, in that it acts as an adjuvant to a model antigen such as ovalbumin (OVA) to boost antigen-specific immunity[1]. To enable researchers to design improved devices using PLGA, we are interested in understanding its mechanism by which it acted as an adjuvant in vivo. We hypothesized that PLGA’s adjuvant effect in vivo is primarily mediated by its effects on dendritic cells – the most potent antigen-presenting cells of the immune system – in an antigen-specific manner. Accordingly, we used a CD11c-DTR mouse model[2] in which CD11c+ cells (of which DCs are the largest population) were conditionally ablated by delivering diphtheria toxin. These mice received OVA-laden PLGA scaffolds as dorsal subcutaneous implants while also having received an intravenous injection with fluorescently label OVA-specific CD4+ T-cells. After allowing sufficient time for the interaction each of these components in vivo, we examined mice spleens for clonal expansion of the exogenously delivered T-cells. Our results clearly indicate that the OVA-specific T-cells in the DT-treated CD11c-DTR mice had failed to proliferate in comparison with non-DT treated controls due to the lack of sufficient DCs in vivo to drive antigen presentation, thereby supporting our original hypothesis that PLGA’s adjuvant effect is mediated via DCs.
Materials and Methods:
CD11c-DTR, OT-II and wild-type C57BL/6 mice of 6-8 weeks were purchased from Jackson labs and quarantined for one week prior to any treatment. CD4+ OVA-specific T-cells were isolated from OT-II mice mice and fluorescently labeled with carboxy fluorescein succinimidyl ester (CFSE) (Invitrogen) and injected in mice intravenously. Diphtheria toxin (Sigma) was supplied as 4ng/g[2]of mouse body weight via intravenous tail vein injections once every two days in order to sustain CD11c+ depletion over a 6-day period. PLGA scaffolds were prepared as described before[3]. Briefly, 75:25 pellets were dissolved in dichloromethane and cast into Teflon dishes mixed with dehydrated OVA (Sigma) and 125 – 250um NaCl salt crystals acting as porogens. The scaffolds thus made were washed, dried and cut into 8mm disks and implanted in the dorsal subcutaneous pockets in mice – one scaffold per mouse. Mice were subsequently sacrificed and their splenocytes were isolated and analyzed using flow cytometry.
Results:
The DC depletion experiment was conducted over a 7-day period with the appropriate treatments and days depicted on figure 1
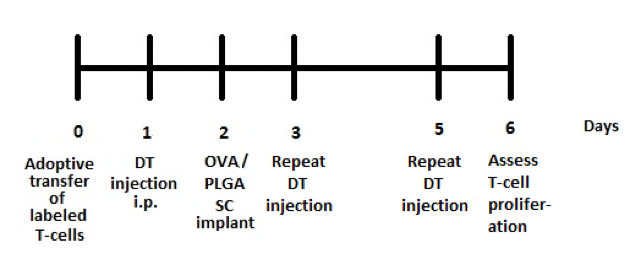
. Splenocytes from 3 mice groups – DTR mice with DT treatment, (control) DTR mice without DT treatment and (control) C57BL/6 wild-type with DT treatment (all with a PLGA/OVA implant– were stained with fluorescent antibodies against specific T-cell markers. CFSE incorporates in the nucleus of cells and is propagated in their daughter cells on cell division, thereby reducing in the extent of fluorescence emitted by the subsequent progeny[2]. Figure 2 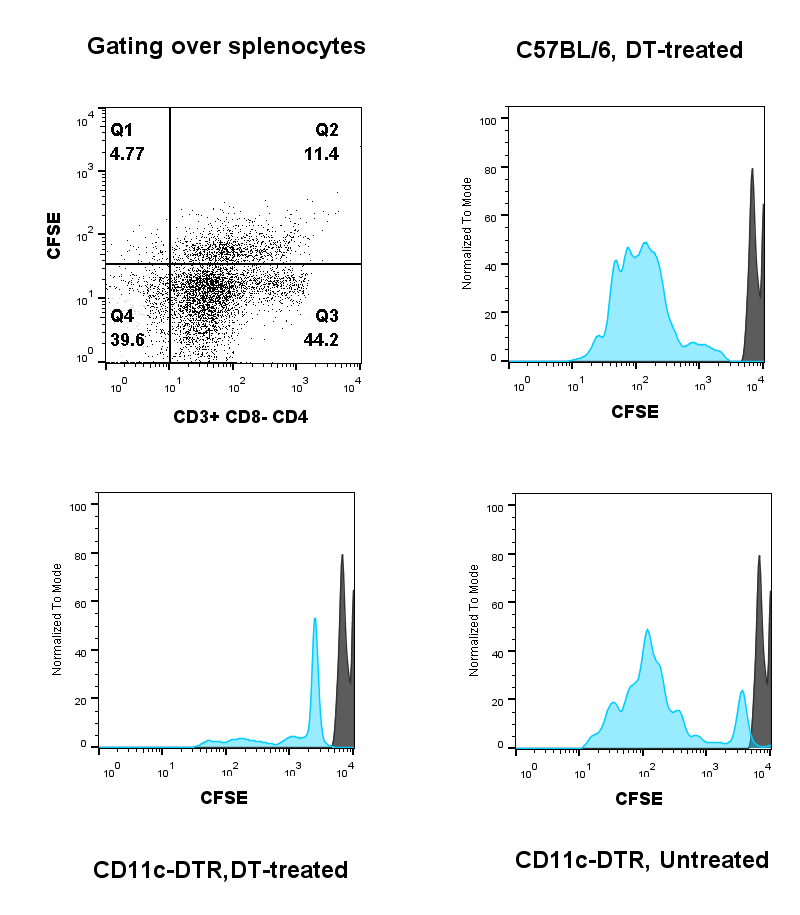
shows the fluorescence intensity of the originally injected cells (shown in black) while that of any of its progeny (shown in blue). The lack of attenuation of CFSE fluorescence signal in DT-treated DTR mice (fig. 2 - bottom right panel) compared to the control groups (fig. 2 – top and bottom left panels) indicate that OVA-specific T-cells were unable to proliferate in DT-treated mice and thereby due to the lack of CD11c+ cells.
National Institute of Health Grant
References:
[1] Norton, L. W. & Babensee, J. E. in Fundamentals of Tissue Engineering and Regenerative Medicine 721–747 (Springer, 2008).
[2] Jung, S. et al. In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity 17, 211–220 (2002).
[3] Park, J. & Babensee, J. E. Differential functional effects of biomaterials on dendritic cell maturation. Acta BIOMATERIALIA 8, 3606–3617 (2012).
Keywords:
in vivo,
biomaterial,
Scaffold,
cell phenotype
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials in immune response
Citation:
Srinivasan
S and
Babensee
JE
(2016). The Adjuvant Effect Of PLGA Is Mediated Via Dendritic Cells In Vivo.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00020
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.